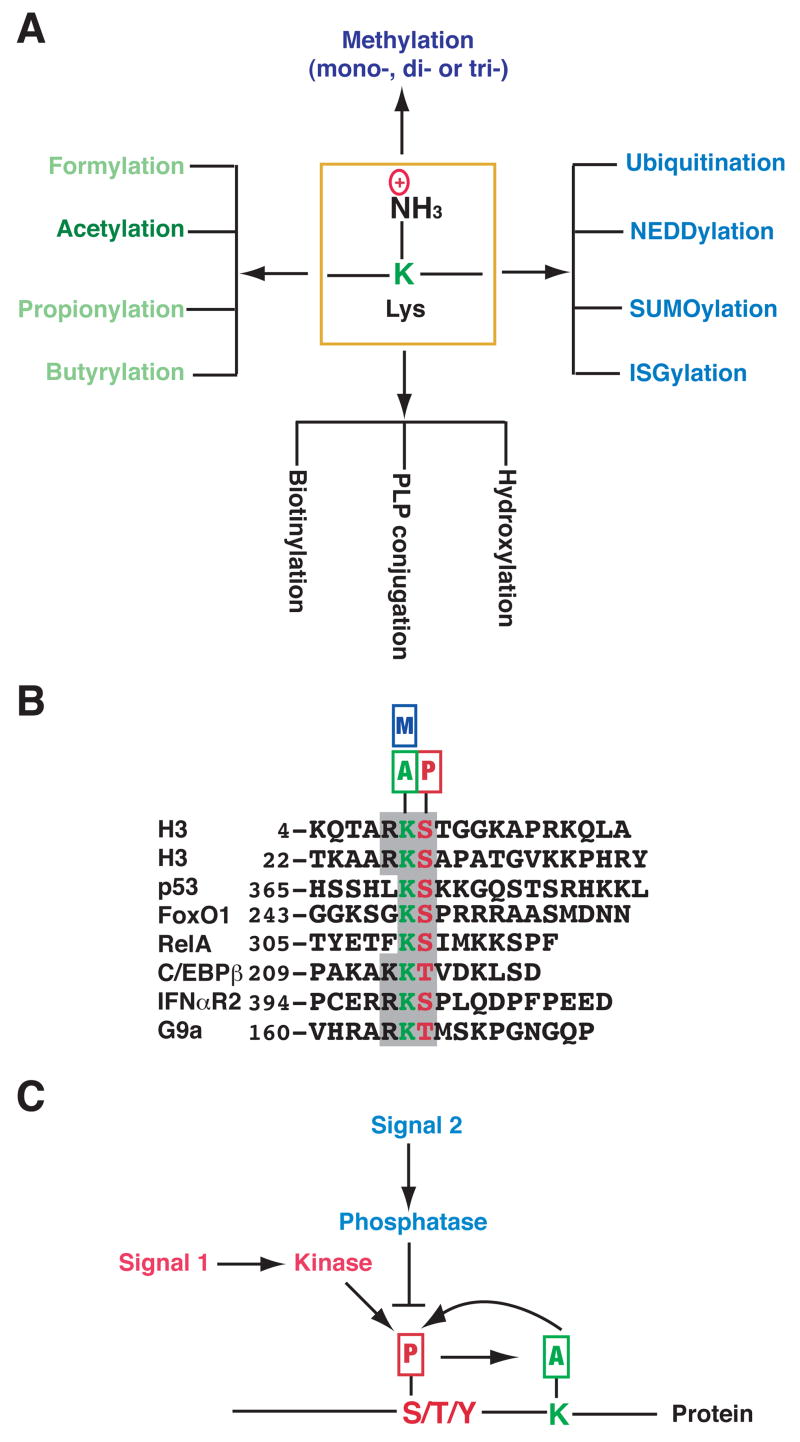
Figure 1. Multiplicity of Lys modifications and signal conversion from phosphorylation to acetylation.
(A) The side chain of a Lys (K) residue can be modified by acetylation and other covalent modifications. Except for hydroxylation, all listed modifications target the ε-amino group. Formylation, propionylation and butyrylation are three recently discovered modifications that are structurally similar to acetylation; however, it is not known if formylation occurs enzymatically. Aside from formylation, hydroxylation and PLP (pyridoxal 5′-phosphate, a vitamin B6 derivative) conjugation, all listed modifications are reversible. Acetylation of a Lys residue precludes further modifications by others, and vice versa.
(B) Alignment of sequences surrounding KS dipeptides from histone H3, p53 and several other proteins. The KS dipeptides are highlighted in color. Small rectangles with the letters A, P and M denote acetylation, phosphorylation and methylation, respectively. IFNαR2 (interferon α receptor 2) acetylation at Lys 399 and phosphorylation at Ser 400 might crosstalk with one another (Tang et al., 2007). It remains to be established whether similar interplay occurs in p53, FoxO1, RelA (NF-κB subunit) (Perkins, 2006), and C/EBPβ (CCAAT/enhancer binding protein β) (Hasselgren, 2007). Methylation of the highlighted Lys residues has been documented only for histone H3, p53 (Huang et al., 2006b) and G9a (Sampath et al., 2007). It is unclear whether the Ser in p53 is phosphorylated or the Lys in G9a is acetylated.
(C) Cartoon illustrating crosstalk between phosphorylation and acetylation. Different signals act on Ser (S), Thr (T) or Tyr (Y) phosphorylation, which in turn affects acetylation of a neighboring Lys. Acetylation might also regulate phosphorylation. The Lys can be adjacent to or far away from the phosphorylation site, which can be either N-terminal or C-terminal from the acetylation site.