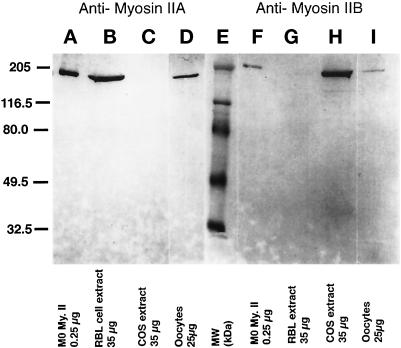
Figure 1.
Antibodies prepared against isoform-specific regions of myosin heavy chains A and B are monospecific and detect 205-kDa proteins in unfertilized mouse oocytes. Lanes A–D, antimyosin IIA antibody. (A) 0.25 μg of purified macrophage (Mφ) myosin II protein; (B) 35 μg of RBL cell extract; (C) 35 μg of COS cell extract; and (D) 25 μg of mouse unfertilized oocyte extract. Lanes F–I, antimyosin IIB antibody. (F) 0.25 μg of purified (Mφ) myosin II protein; (G) 35 μg of RBL cell extract; (H) 35 μg of COS cell extract; and (I) 25 μg of mouse unfertilized oocytes extract. Lane E, molecular mass standards, in kDa. Myosin IIA antibody recognizes a 205-kDa myosin IIA isoform in RBL cell extracts, but not extracts from COS cells which lack myosin IIA. In contrast, the 205-kDa myosin IIB isoform is detected in COS cell extracts, but is absent in RBL cell extracts which lack the IIB protein. Myosin IIA and IIB antibodies detect 205-kDa proteins in mature oocytes. The IIB isoform appears to be less prevalent in oocytes than myosin IIA.