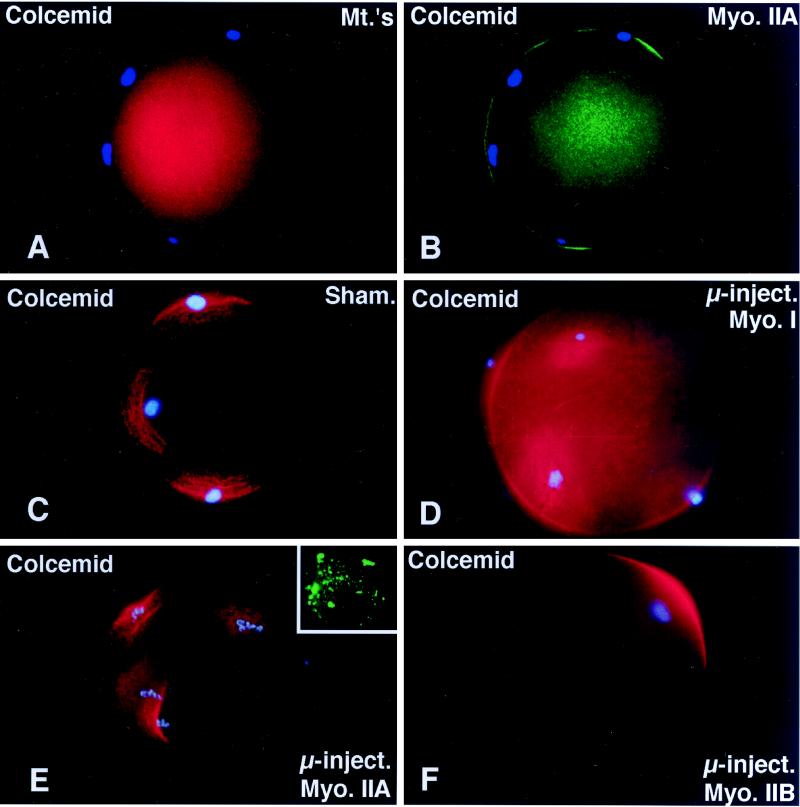
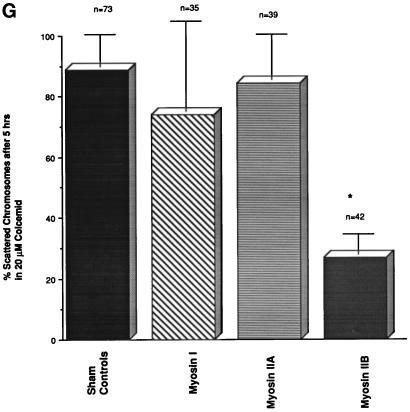
Figure 4.
Microinjected myosin IIB antibody blocks microfilament-mediated chromosome scattering following Colcemid-induced disassembly of the second meiotic spindle. (A and B) Unfertilized oocytes incubated in 20 μM Colcemid for 5 h disassemble the meiotic spindle (A, red), resulting in meiotic chromosome scattering (A, blue). Meiotic chromosome scattering induces an increase in cortical myosin IIA (B, green) at sites adjacent to, but often not over, each set of chromosomes (B, blue). (C–E) Mature unfertilized oocytes which were sham-treated (C) and microinjected with myosin I (D) or myosin IIA (E) antibodies. After 5 h in 20 μM Colcemid, all three sets of oocytes demonstrate dispersed cortical chromosomes and an increase in actin accumulation (red) in the plasma membrane regions adjacent to the scattered chromosome masses (blue). Inset in E, microinjected myosin IIA labeling of the scattered meiotic chromosomes. (F) Microinjection of myosin IIB antibody blocks chromosomal dispersion in Colcemid-treated unfertilized oocytes. A single region of enhanced cortical actin overlies the intact chromosomes. (G) To quantify the effects of myosin antibodies on meiotic chromosome scattering following Colcemid-induced spindle disassembly, unfertilized oocytes were microinjected with myosin I, myosin IIA, or myosin IIB antibodies before placement into Colcemid for 5 h. The percentage of scattered chromosomes with overlying enhanced cortical actin was then recorded. The graph demonstrates that chromosome scattering is significantly reduced in the presence of myosin IIB antibody but not by the microinjection of myosin I or IIA antibodies. ∗, significant difference with sham-microinjected oocytes (p < 0.01). (A and B) Triple labeled for myosin IIA (green), microtubules (red), and DNA (blue). (C–F) Double labeled images for actin (red) and DNA (blue). Bar, 10 μm.