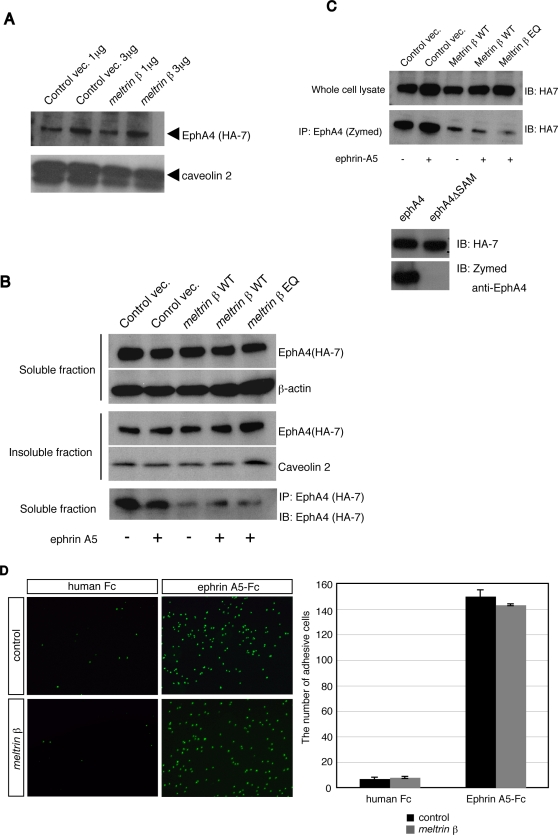
Figure 5. Meltrin β does not affect the expression of EphA4 on the cell surface nor the initial association between ephrin-A5 and EphA4, but decreases accessibility of antibodies that recognize the EphA4 C-terminus.
(A) EphA4-HA transformant cells were transfected with Meltrin β expression plasmids (meltrin β) or with control vectors, and biotinylated proteins on the cell surface were precipitated with streptavidin beads. The proteins were separated by SDS-PAGE, and EphA4 was detected with an anti-HA antibody (HA-7). Caveolin 2 was used as an internal control. (B) NIH3T3 cells were transfected with EphA4-HA expression plasmids and the various plasmids listed at the top of the lanes and stimulated with ephrin-A5-Fc fusion proteins as indicated. The cell lysates were divided into a Triton-X100–solubilized fraction (soluble fraction) and Triton-X100–resistant raft microdomains (insoluble fraction). After SDS-PAGE separation, EphA4 was detected with HA-7. EphA4 proteins in the soluble fractions were also immunoprecipitated with HA-7 (the bottom panel) and detected with HA-7 after SDS-PAGE. The amount of EphA4 precipitated was reduced dramatically when the Meltrin β WT or EQ mutant was coexpressed with EphA4, regardless of stimulation with ephrin-A5. (C) (Upper Panel) NIH3T3 cells were transfected with EphA4-HA expression plasmids and the various plasmids listed at the top of the lanes and stimulated with ephrin-A5-Fc fusion proteins as indicated. EphA4 proteins in the soluble fractions (whole cell lysate) were immunoblotted with anti-HA antibody (HA-7) or immunoprecipitated with an anti-EphA4 antibody and detected with HA-7 after SDS-PAGE. Immunoprecipitation of EphA4 with anti-EphA4 was inefficient when the meltrin β WT or EQ mutant were coexpressed, regardless of the stimulation with ephrin-A5-Fc. (Lower Panel) An anti-EphA4 antibody from Zymed recognizes the SAM domain in the EphA4-C terminus. NIH3T3 cells were transfected with plasmids that express EphA4-HA or SAM domain–deleted EphA4-HA, and expressed proteins were detected either with HA-7 or with the anti-EphA4 antibody after SDS-PAGE of the cell lysates. (D) EphA4 transformants transfected with meltrin β or a control vector were seeded onto plastic dishes coated with a human Fc fragment or ephrin-A5-Fc. The binding activity of EphA4-expressing cells (detected with DAPI) on ephrin-A5-coated plates was not changed by coexpression of Meltrin β.