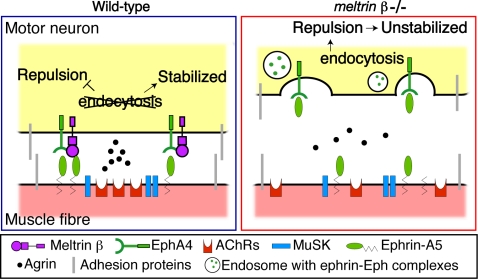
Figure 7. Hypothetic model of how Meltrin β and ephrin-A5–EphA4 signaling stabilize the NMJ.
When axon terminals touch the ephrin-A5-expressing postsynaptic region, EphA4 associated with Meltrin β is localized preferentially at the axon terminal opposite the postsynaptic cluster on the muscle fiber; Meltrin β interferes with the internalization of the ephrin-A5–EphA4 complexes so that the axon terminal and muscle could avoid receiving a repulsive signal at the NMJ, and many molecules, such as agrin, MuSK and adhesion proteins, cooperate in stabilization of the NMJ. In the absence of Meltrin β, axon terminals of motor neurons cannot be stabilized because impaired suppression of endocytosis would result in a failure to block the ephrin-A5–EphA4 repulsive signal. As a result, the motor terminals remain mobile at the NMJ, and the reverse signal from EphA4 to ephrin-A5 is unstable, leading to the diffuse distribution of ephrin-A5 and AChR transcripts in muscles. (see also text).