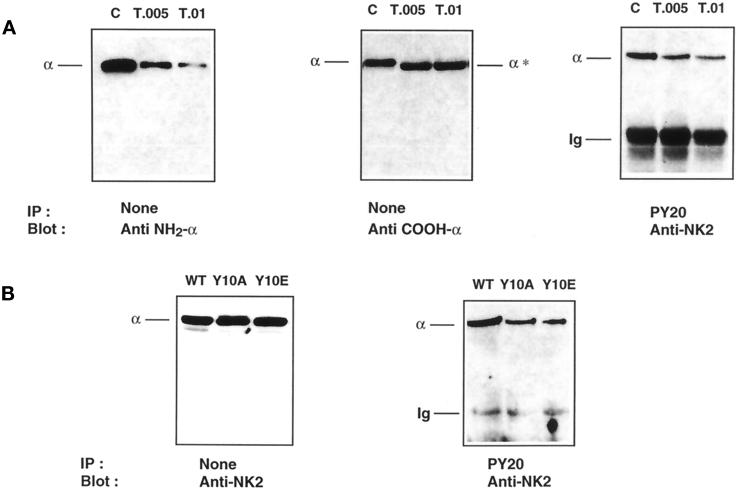
Figure 6.
The Na+, K+-ATPase α-subunit is phosphorylated at tyrosine 10. (A) Proximal tubules were preincubated for 30 min at 37°C with 10−8 M insulin, and 200 μg protein were incubated for 5 min at 4°C without (C) or with trypsin (T) at a trypsin:protein ratio (wt/wt) of 0.005–0.01. Immunoblots of total protein with McK1 antibody (Anti NH2-α; left panel) and with an antibody raised against the extreme COOH-terminal ETYY motif (Anti COOH-α; middle panel) show that tryptic cleavage of the α-subunit has occurred exclusively at the extreme NH2 terminus. This limited trypsinolysis removed the tyrosine phosphorylation site recognized by PY20 antibody (right panel). Results are representative of four independent experiments. (B) OK cells were stably transfected with the WT rat α1-subunit (WT) or its Y10A or Y10E mutants. Immunoblot of total protein (left panel) shows that transfected cell lines express similar amounts of α-subunit, but substitution of Tyr-10 by Ala or Glu greatly reduces the amount of α-subunit immunoprecipitated by PY20 antibody (right panel).