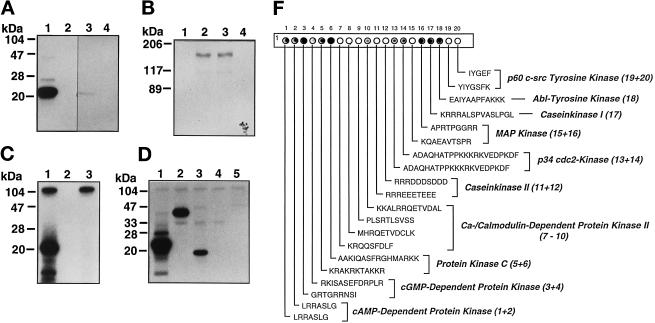
Figure 4.
Protein kinase activity of ANPK. CHO cells were transiently transfected with 10 μg of expression vectors for FLAG-tagged ANPK(159–1191) and ANPK(K226R), in which Lys226 in the ATP-binding site was converted to an Arg. Proteins were immunoprecipitated using anti-FLAG mAb and subjected to immune complex kinase assays. (A) Phosphorylation of myelin basic protein (MBP, 5 μM) by immunopurified ANPK(159–1191) (lane 1), but not by ANPK(K226R) (lane 3), ANPK(159–1191), and ANPK(K226R) in the absence of MBP (lanes 2 and 4, respectively). (B) Autophosphorylation by ANPK(159–1191) (lanes 2 and 3) and ANPK(K226R) (lanes 1 and 4). (C) Protein kinase activity of purified GST-ANPK(159–920) expressed in E. coli. GST-ANPK(159–920) with MBP (lane 1), autophosphorylation of GST-ANPK(159–920) in the absence of MBP (lane 3), and MBP in the absence of GST-ANPK(159–920) (lane 2). (D) Comparison of MBP (lane 1), c-Jun (lane 2), histone H3 (lane 3), histone H1 (lane 4), and GST-ANPK(802–920) (lane 5) as substrates for GST-ANPK(159–920). Reaction mixture (20 μl) contained 0.7 μg of GST-ANPK(159–920) and 5 μM substrate. After 30°C for 30 min, the reactions were terminated by addition of 20 μl of 2× Laemmli sample buffer, after which the products were subjected to electrophoresis under denaturing conditions on 15% (A, C, and D) or 7.5% polyacrylamide gels (B) and visualized by autoradiography. (F) Solid-phase phosphorylation of synthetic peptides by ANPK. PhosphoSpots test strip (Jerini Bio Tools) containing covalently bound substrate peptides for indicated kinases was phosphorylated by incubating with 4 μg of GST-ANPK(159–920) in 500 μl of 2× kinase buffer containing 100 μM [γ-32P]ATP at 22°C for 20 min. The reaction was stopped and filter washed extensively according to manufacturer’s instructions and analyzed by autoradiography. (The peptide no. 19 contains both Ser and Tyr residues as potential phosphoacceptor sites.)