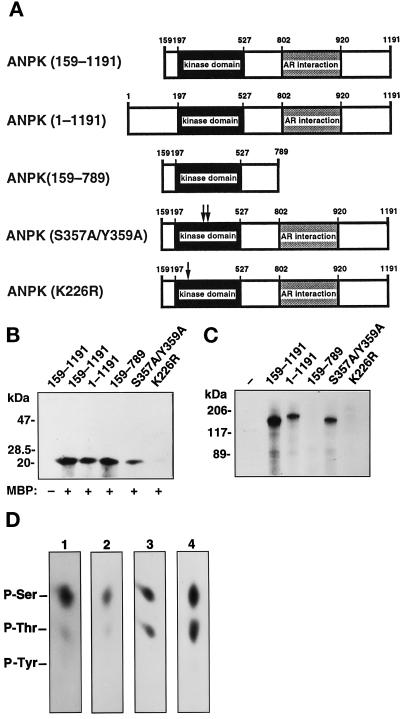
Figure 6.
Protein kinase activity and autophosphorylation of different ANPK mutants. (A) Structural features of ANPK mutants studied. (B) Protein kinase activity of ANPK mutants. FLAG-tagged ANPK and indicated mutant proteins were expressed in CHO cells, immunoprecipitated using anti-FLAG monoclonal antibody, and subjected to immune complex kinase assays for 30 min at 30°C in the absence (–) and presence (+) of 5 μM MBP as described in MATERIALS AND METHODS. (C) Immune complex kinase assays of various ANPK forms in the absence of added substrate. Different ANPK forms were expressed to similar levels as judged by immunoblotting analysis (our unpublished data). (D) Phosphoamino acid analysis of autophosphorylated ANPK and MBP and c-Jun phosphorylated by ANPK in vitro. After in vitro kinase assay using immunopurified ANPK (1) and ANPK(S357A/Y359A) (2) from CHO cells, or MBP (3) and c-Jun (4) as substrates in the GST-ANPK(159–920) catalyzed phosphorylation, proteins were transferred onto PVDF membrane, radioactive bands corresponding to phosphorylated proteins were cut out and subjected to acid hydrolysis as described in MATERIALS AND METHODS. Phosphoamino acids were resolved by one-dimensional electrophoresis at pH 2.5. Positions of unlabeled internal phosphoamino acid standards are depicted.