Table 3. Substrate specificity of TbEK1 (formerly TbC/EK1) and TbC/EK2 to utilize analogues modified by extra substitutents on the carbon backbone of the acceptor substrate.
Results are means±S.D. (n≥3).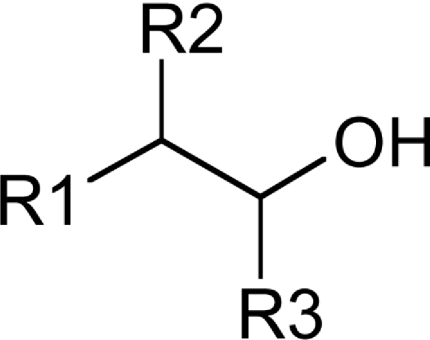
| R1 | R2 | R3 | Compound | TbEK1 relative rate* | TbC/EK2 relative rate† |
|---|---|---|---|---|---|
| -N+(CH3) 3 | -H | -H | Choline | 0.03±1.59 | 100.00±7.97 |
| -NH2 | -H | -H | Ethanolamine | 100.00±1.65 | 33.34±3.34 |
| -NH2 | -CH3 | -H | R(−)-2-Aminopropan-1-ol | 107.91±2.77 | 109.94±15.42 |
| -NH2 | -CH3 | -H | S(+)-2-Aminopropan-1-ol | 93.75±4.02 | 22.38±1.87 |
| -NH2 | -(CH3)2 | -H | 2-Amino-2-methylpropan-1-ol | 25.58±3.98 | 46.41±6.57 |
| -NH2 | -CH2CH3 | -H | S(+)-2-Aminobutan-1-ol | 114.07±5.84 | 40.72±4.93 |
| -NH2 | -CH2CH3 | -H | R(−)-2-Aminobutan-1-ol | 101.74±5.76 | 130.40±9.10 |
| -NH2 | -COOH | -H | L-Serine | 0.94±2.25 | 0.00±0.00 |
| -NH2 | -H | -CH3 | R(−)-1-Aminopropan-2-ol | 52.41±4.50 | 7.16±0.60 |
| -NH2 | -H | -CH3 | S(+)-1-Aminopropan-2-ol | 6.07±2.13 | 1.37±0.15 |
| -CH2NH2 | -H | -H | 3-Aminopropan-1-ol | 37.52±2.96 | 44.52±4.33 |
| (CH2)2NH2 | -H | -H | 4-Aminobutan-1-ol | 0.05±1.93 | 59.95±6.00 |
*Analogues are compared with ethanolamine at the constant concentration of 1 mM and a saturating concentration of ATP to give a relative rate (ethanolamine=100).
†Analogues are compared with choline at the constant concentration of 1 mM and a saturating concentration of ATP to give a relative rate (choline=100).
