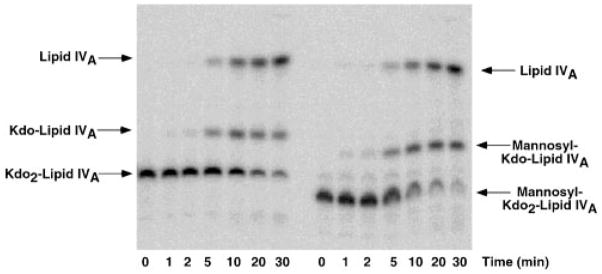
Fig. 6. Time course of hydrolysis at 100 °C of Kdo2-[4′-32P]lipid IVA versus mannosyl-Kdo2-[4′-32P]lipid IVA.
A, the Kdo2-[4′-32P]lipid IVA control. B, mannosyl-Kdo2-[4′-32P]lipid IVA. The hydrolysis is carried out in sodium acetate buffer at pH 4.5 in the presence of SDS (19). The two Kdo glycosidic linkages are about equally susceptible to cleavage under these conditions, allowing discrimination between mannose addition to the outer versus the inner Kdo (19). LpcC modifies the inner Kdo as shown by the absence of unmodified Kdo-[4′-32P]lipid IVA during the time course of the hydrolysis of the mannosyl-Kdo2- [4′-32P]lipid IVA.