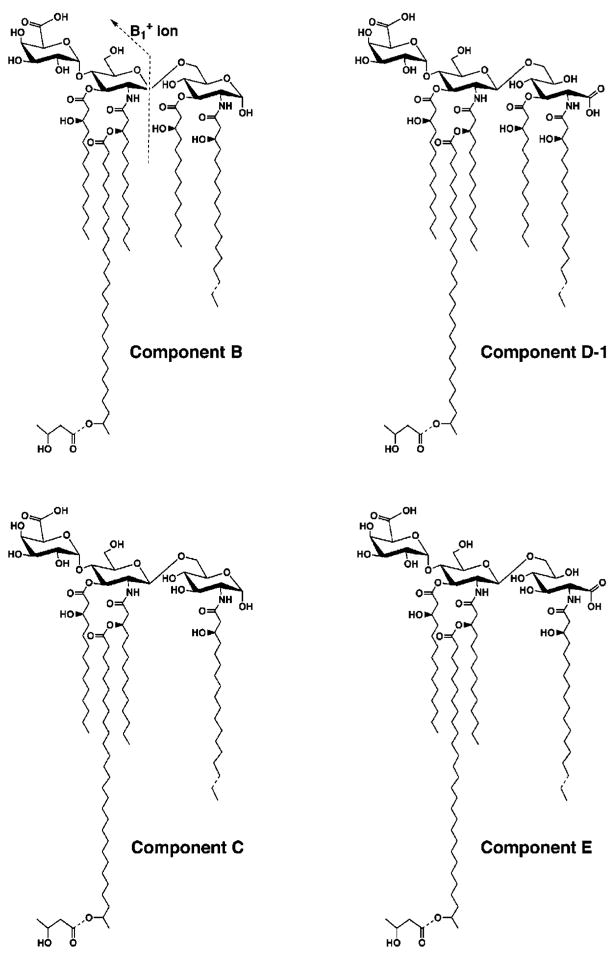
Fig. 10. Proposed covalent structures of components B, C, D-1, and E of R. etli CE3 lipid A.
Components B and C contain a glucosamine disaccharide unit, typical of most known lipid A molecules, including that of E. coli. Evidence for the β, 1′-6 linkage in B and C, and for the absence of an acyl chain at position 3 in C, is provided by the NMR studies in the accompanying article (73). Components D-1, D-2 (not shown), and E are proposed to contain an aminogluconate residue in place of the proximal glucosamine. D-1 and D-2 have the same molecular masses (as judged by the MALDI/TOF analysis), but they appear to be interconverted during prolonged incubation at room temperature in aqueous buffers and organic solvents. This process probably results from the migration of the fatty acyl at the C-3 position in D-1 to the C-5 position of the aminogluconate residue to generate D-2 (not shown). Acyl chain migration is known to occur in mono-acylated derivatives of UDP-N-acetylglucosamine (54), which are early intermediates in the lipid A pathway. The predominant substances that are isolated from cells are B and D-1/D-2. All components, including A, have the same distal unit, as indicated by the common ion fragments in Figs. 6 and 11. Dashed bonds indicate microheterogeneity with respect to acyl chain lengths and the presence or absence of the β-hydroxybutyrate substituent. The molecular weights calculated in Table I are those of the species with the longer acyl chains at position 2 and those that are decorated with the β-hydroxybutyrate group.