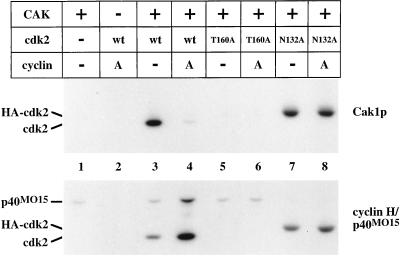
Figure 2.
Phosphorylation of cdk2. Wild-type cdk2 expressed in insect cells and mutant forms of cdk2 expressed in bacteria were incubated in the presence of radiolabeled ATP with GST-Cak1p or p40MO15/cyclin H. Samples were analyzed on 10% SDS-PAGE for incorporation of radiolabeled phosphate by autoradiography. T160A contains a mutation of the activating phosphorylation site (lanes 5 and 6), whereas N132A renders cdk2 catalytically inactive (lanes 7 and 8). Cyclin A173–432 was present in the indicated lanes in a threefold mass excess over cdk2. The mobility of cdk2N132A is decreased due to the presence of an N-terminal influenza hemagglutinin (HA) epitope tag (lanes 7 and 8). A low level of phosphorylation of p40MO15 by active cdk2 complexes is apparent in lane 4 (see also Fisher et al., 1995).