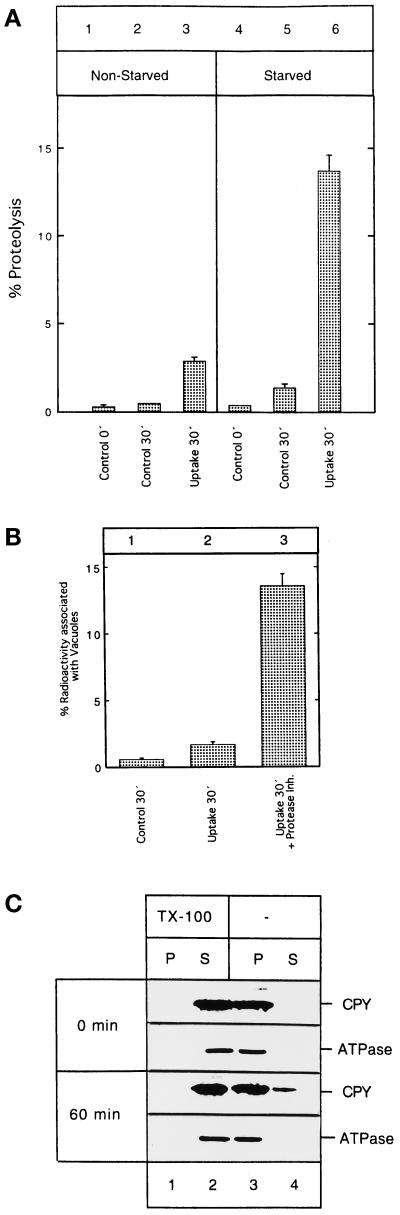
Figure 2.
Cytosolic proteins are imported into and degraded by isolated vacuoles. (A) Vacuoles were isolated from yeast cells grown in either YPD (lanes 1–3) or nitrogen starvation medium (lanes 4–6). After 30 min incubation at 30°C with a cytosolic extract prepared from [3H]leucine-labeled cells, uptake reactions were terminated by adding TCA (lanes 3 and 6). To control for the integrity of the isolated vacuoles during the incubation period, vacuoles were incubated for 30 min at 30°C and pelleted, and the supernatant was incubated for an additional 30 min at 30°C with radiolabeled cytosol before the reaction was stopped with TCA (lanes 2 and 5). Background caused by lysed vacuoles and proteins already degraded in the cytosolic extract at the beginning of the incubation period was measured by TCA-precipitating samples at time 0 min (lanes 1 and 4). Radioactivity of the acid-soluble material in all samples was determined by liquid scintillation counting. Data and SD was calculated from three independent experiments performed in duplicate. (B) Vacuoles were isolated from yeast cells grown in nitrogen starvation medium. After a 30-min incubation at 30°C with a radiolabeled cytosolic extract, uptake reactions were terminated by pelleting the vacuoles. Vacuoles were resuspended in import buffer, and cytosolic proteins associated with the surface of the vacuoles were digested by externally added proteinase K for 30 min on ice. Proteinase K treatment was stopped by adding PMSF, and proteins were TCA-precipitated. Radioactivity associated with the pellet was determined by liquid scintillation counting (lane 2). In one sample, vacuolar proteases were inhibited by a 10-min pretreatment of the isolated vacuoles with the protease inhibitors E-64 (100 μM), leupeptin (20 μM), and pepstatin (20 μM) on ice (lane 3). To determine the background caused by the presence of proteinase K-resistant proteins on the vacuolar surface, vacuoles were incubated with the cytosol for 30 min on ice, vacuoles were reisolated by centrifugation, proteinase K-treated, and TCA-precipitated, and the radioactivity of the pellet was determined by liquid scintillation counting (lane 1). Data and SD were calculated from two independent experiments performed in duplicate. (C) Latency of the vacuoles isolated from starved cells was measured at 0 min and after 1 h at 30°C. Samples containing vacuoles plus cytosolic extracts were split into two aliquots. They were incubated with or without 1% Triton X-100 (TX-100; −) for 10 min on ice and pelleted, and the pellets were thoroughly resuspended in 1% TX-100 containing buffer. The proteins in the pellets (P) and supernatants (S) were TCA-precipitated, separated by SDS-PAGE, and immunoblotted with antibodies against carboxypeptidase Y and the 100-kDa subunit of the Fo complex of the vacuolar ATPase as a control.