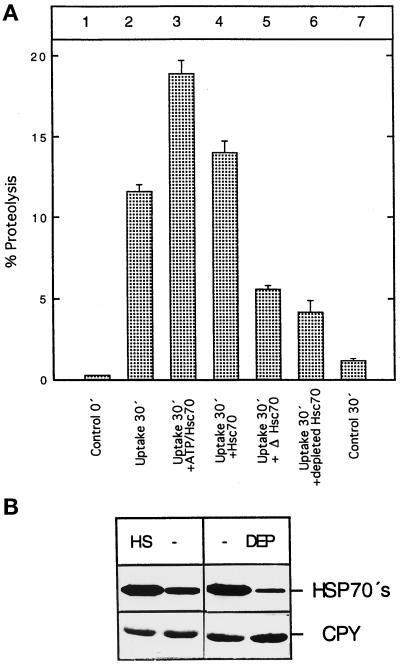
Figure 5.
Uptake of cytosolic proteins depends on cytosolic hsp70s. (A) Vacuoles were isolated from yeast cells grown in nitrogen starvation medium. Uptake was performed as described in the legend to Figure 2A, except that different cytosolic extracts from [3H]leucine-labeled cells were used (lanes 2–6). For the reaction in lane 2, a cytosolic extract from wild-type cells grown at 30°C was used; for the sample in lane 3, a cytosolic extract from cells heat-shocked for 2 h at 37°C was used together with an ATP-regenerating system; in lane 4 the same cytosol was used as in lane 3 except that an ATP-regenerating system was not present; for the reaction in lane 5, a cytosol from cells in which the genes for the two constitutively expressed cytosolic hsp70s in yeast had been deleted was used; for the reaction in lane 6, a cytosol immunodepleted with an antiserum recognizing Ssa1, Ssa2, and Ssa3 was used. Radioactivity of the acid-soluble material was determined by liquid scintillation counting. Data and SD were calculated from two independent experiments performed in triplicate. (B) The amounts of cytosolic hsp70s in the cytosol prepared from heat-shocked cells (HS) compared with non–heat-shocked cells (−) and the amount of cytosolic hsp70s in immunodepleted cytosol (DEP) compared with control cytosol (−) were estimated by Western blot analysis using an antiserum that recognized Ssa1, Ssa2, and Ssa3 (HSP70s; E. A. Craig, personal communication). A Western blot with an antiserum raised against carboxypeptidase Y (CPY) was used to check whether the same amount of protein was loaded in each lane of the gel.