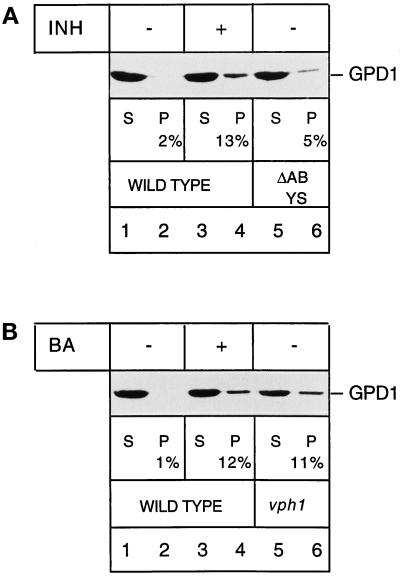
Figure 7.
Glyceraldehyde-3-phosphate dehydrogenase is imported into and degraded by isolated vacuoles. (A) Wild-type cells (lanes 1–4) and cells in which four major vacuolar proteases had been deleted (ΔABYS, lanes 5 and 6) were grown in nitrogen starvation medium. Vacuoles were prepared from both strains. In the uptake shown in lanes 3 and 4, the isolated vacuoles were pretreated for 10 min on ice with E-64 (100 μM), leupeptin (20 μM), and pepstatin (20 μM) (INH) to block vacuolar protein degradation. Cytosolic extract from wild-type cells was added to the vacuoles at 30°C for 30 min, and the uptake was terminated by pelleting the vacuoles. The supernatant was TCA-precipitated, and 50% of the supernatant (S) was separated by SDS-PAGE and immunoblotted for GPD1 (lanes 1, 3, and 5). The vacuolar pellet was resuspended in import buffer, and cytosolic proteins associated with the surface of the vacuoles were digested by externally added proteinase K (100 μg/ml) for 30 min on ice. The digest was stopped by adding PMSF, and proteins were TCA-precipitated (P), separated by SDS-PAGE, and immunoblotted for GPD1 (lanes 2, 4, and 6). The percent values given in the figure correspond to the amount of GPD1 associated with the vacuolar fraction. (B) Wild-type cells (lanes 1–4) and cells in which the 100-kDa subunit of the vacuolar ATPase subcomplex Fo had been deleted (vph1, lanes 5 and 6) were grown in nitrogen starvation medium. Vacuoles were prepared from both strains. In the uptake shown in lanes 3 and 4, the isolated wild-type vacuoles had been pretreated for 10 min on ice with 1 μM bafilomycin (BA), an inhibitor of the vacuolar ATPase. The uptake, the proteinase K treatment, and the processing of the samples were performed as described in A.