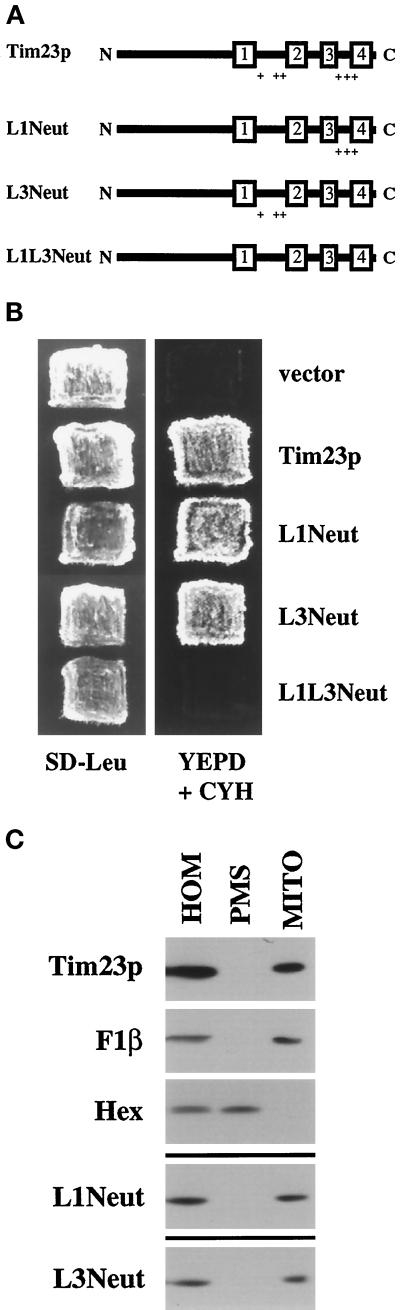
Figure 2.
One of two sets of positively charged segments within Tim23p is required for function, but not for targeting to mitochondria in vivo. (A) Schematic representation of the Tim23 protein and mutant Tim23p constructs used in this study. The numbered rectangles represent the four hydrophobic regions corresponding to proposed membrane-spanning segments. The + signs denote positively charged amino acids within the loops (L1 or L3) between the TM segments. (B) Positively charged amino acids in either segment L1 or L3 are needed for Tim23p function. tim23::URA3 trp1 leu2 cyh2 strain KRR146 carrying TIM23-TRP1-CYH2 plasmid pKR1 was transformed with either the LEU2 vector pRS315 or pRS315 carrying DNA inserts encoding Tim23p (pJE50), L1Neut (pAD62), L3Neut (pAD58), or L1L3Neut (pAD64). Leu+ colonies were patched out onto synthetic complete medium lacking leucine (SD−Leu). Patches were grown at 30°C for 2 d, and then replica plated onto YEPD medium containing 10 mg/L cycloheximide (YEPD + CYH). Yeast cells that contain a functional Tim23 protein are able to lose the TIM23-TRP1-CYH2 plasmid and grow in the presence of cycloheximide. (C) Tim23p lacking positive charges in segment L1 or L3 are still targeted to mitochondria in vivo. tim23::URA3 trp1 leu2 cyh2 strain KRR146 containing plasmids expressing Tim23p (pJE50), L1Neut (pAD62), or L3Neut (pAD58) were homogenized (HOM) and separated into a mitochondrial pellet (MITO) and a postmitochondrial supernatant (PMS). Proteins from the cell fractions representing equivalent cell amounts were analyzed by SDS-PAGE and immune blotting with antisera to Tim23p, the β-subunit of the F1-ATPase (F1β, a mitochondrial marker), or hexokinase (Hex, a cytoplasmic marker). Immune blots of fractions from cells expressing L1Neut and L3Neut decorated with antiserum to F1β and hexokinase were identical to those shown for Tim23p cells and are not shown.