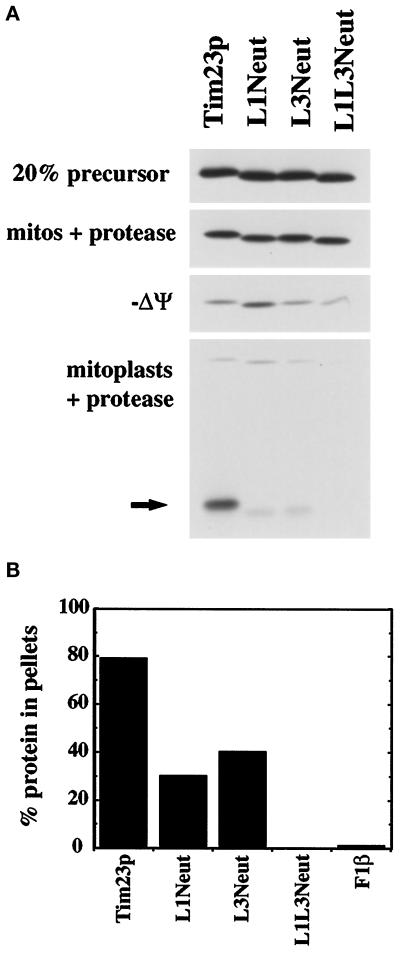
Figure 3.
Internal positively charged segments mediate the insertion of Tim23p into the IM but are not required for import into mitochondria. (A) The Tim23, L1Neut, L3Neut, and L1L3Neut proteins were synthesized in the presence of 35S-methionine and imported into isolated mitochondria as described in MATERIALS AND METHODS. To dissipate the IM potential (−Δψ), mitochondria were preincubated with 250 mM KCl and 40 μM valinomycin. After import, mitochondria were treated with 200 μg/ml trypsin, split into aliquots, and reisolated by centrifugation. Two sets of samples were resuspended in SDS-sample buffer (mitos + protease; −Δψ mitos + protease). In the other samples, the OM was disrupted by OS, and the resulting mitoplasts were treated with 50 μg/ml proteinase K. Mitoplasts were isolated by centrifugation and resuspended in SDS-sample buffer (mitoplasts + protease). Proteins were separated by SDS-PAGE, and radiolabeled proteins were visualized by fluorography; 20% of the translation product used in the import reactions is also shown. The arrow indicates the 14-kDa fragment of Tim23p protected from protease digestion in mitoplasts, indicative of the proper insertion of Tim23p into the IM. (B) Radiolabeled Tim23, L1Neut, L3Neut, L1L3Neut, and F1β proteins were imported into mitochondria and treated with 200 μg/ml proteinase K. Mitochondria were isolated by centrifugation, and the pellets were resuspended in 0.1 M sodium carbonate, pH 11.4. After incubation on ice for 30 min, samples were spun at 100,000 × g for 30 min at 4°C. Pellets and supernatants were subjected to SDS-PAGE, fluorography, and densitometry. The amount of the Tim23, L1Neut, L3Neut, L1L3Neut, and F1β proteins found in the pellet fraction is indicated.