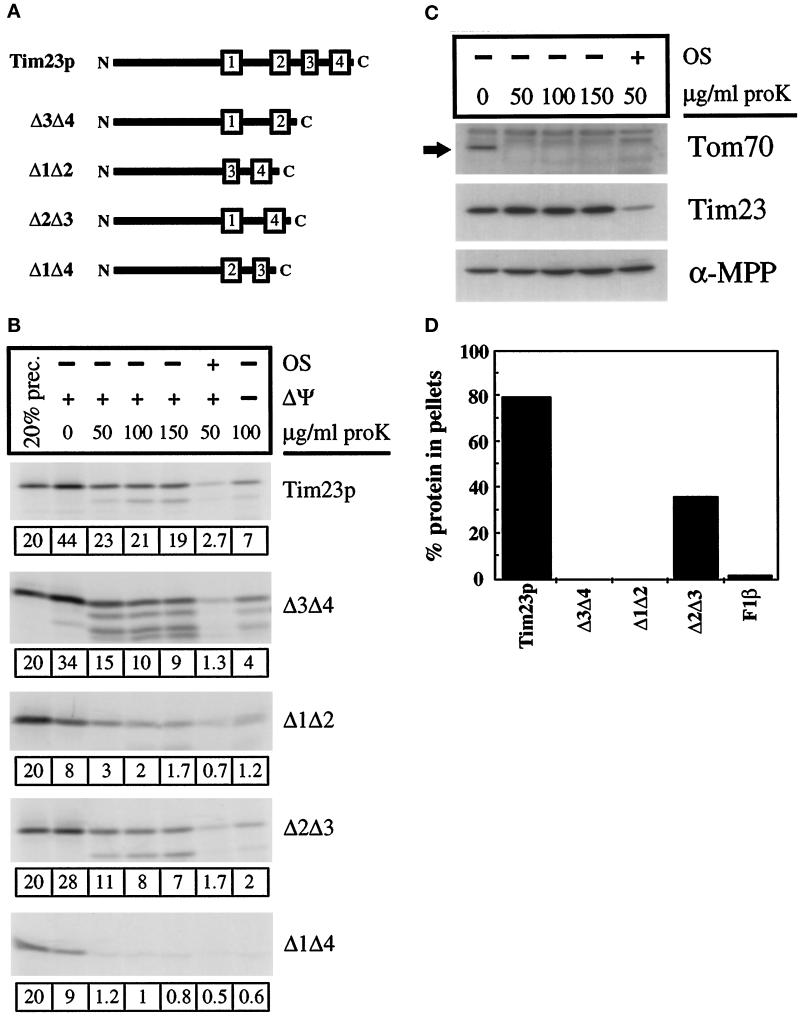
Figure 5.
The hydrophobic carboxyl terminus of Tim23p carries redundant targeting information. (A) Schematic representation of Tim23p deletion constructs used in this study. The numbered rectangles represent the hydrophobic regions corresponding to proposed membrane-spanning segments within Tim23p. All constructs carry the 96-amino acid amino-terminal domain of Tim23p. Δ3Δ4, Δ1Δ2, and Δ1Δ4 all retain the normal loops between the TM segments, whereas Δ2Δ3 contains a hybrid loop (see MATERIALS AND METHODS). (B) Tim23, Δ1Δ2, Δ3Δ4, Δ2Δ3, and Δ1Δ4 imports. Radiolabeled proteins were imported into isolated mitochondria. For one set of samples, the IM potential was dissipated with valinomycin before import (−ΔΨ). After import, samples were split into aliquots and treated with the indicated amounts of proteinase K. In another set of imports, mitochondria were converted to mitoplasts by osmotic shock (OS), followed by protease treatment. Mitochondrial pellets were analyzed by SDS-PAGE and fluorography. Twenty percent of the translation product used in the import reactions is also shown. The amount of the imported protein, calculated as a percentage of the total material added to the import reaction, is shown below each gel. (C) Immune blots. Mitochondria were subjected to mock import conditions and subsequent protease treatment or OS and protease treatment, concurrently with import samples in Figure 5B. Mitochondria or mitoplasts were pelleted and proteins were analyzed by SDS-PAGE and immune blotting with antisera against Tim23p, α-MPP (a matrix marker), and Tom70p (an OM marker). (D) Radiolabeled Tim23, Δ3Δ4, Δ1Δ2, Δ2Δ3, and F1β proteins were imported into mitochondria and treated with 200 μg/ml proteinase K. Mitochondria were isolated by centrifugation, and the pellets were resuspended in 0.1 M sodium carbonate (pH 11.4). After incubation on ice for 30 min, samples were spun at 100,000 × g for 30 min at 4°C. Pellets and supernatants were subjected to SDS-PAGE, fluorography, and densitometry. The amount of the Tim23, Δ3Δ4, Δ1Δ2, Δ2Δ3, and F1β proteins found in the pellet fraction is indicated.