FIGURE 1.
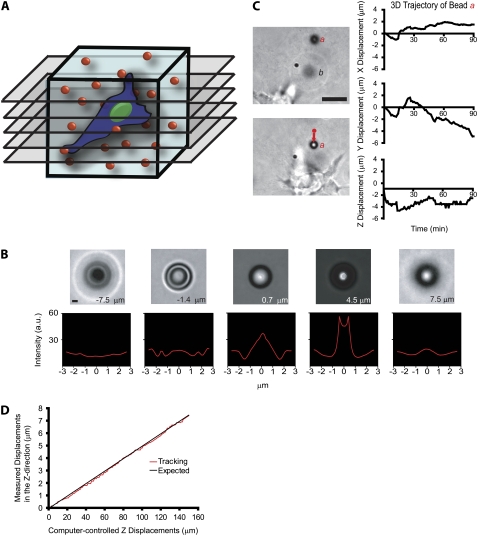
Method to probe the local 3D deformation of the 3D matrix induced by a migratory cell. (A) Schematic of the method used to quantify the local deformation of a 3D collagen matrix (light blue) during cell migration. 4D bright-field microscopy is used to track in equally spaced planes of focus the time-dependent 3D movements of individual 3.6-μm-diameter carboxylated polystyrene beads dispersed in the matrix with high x, y, and z resolution. (B) To calibrate bead movements and before each experiment, randomly selected beads in the matrix are moved by the motorized microscope stage in the z direction to generate a reference image set. High z-movement resolution is achieved by analyzing the diffraction rings of the bead (see text for details). Scale bar, 3 μm. (C) Typical x, y, and z movements of a single polystyrene bead a near a migrating HT-1080 cancer cell (left panel). The initial coordinates of bead a were subtracted (right panel). Bead b is an example of a bead that becomes out of focus, illustrating the 3D deformation of the matrix. Scale bar, 20 μm. (D) Typical vertical displacement of a bead embedded in the matrix (i.e., “output” displacement) as a function of the computer-imposed vertical displacement of this bead by the microscope stage (i.e., “input” displacement). The root mean-squared difference between these two displacements is 120 nm, the spatial resolution of our particle-tracking method in the z direction.