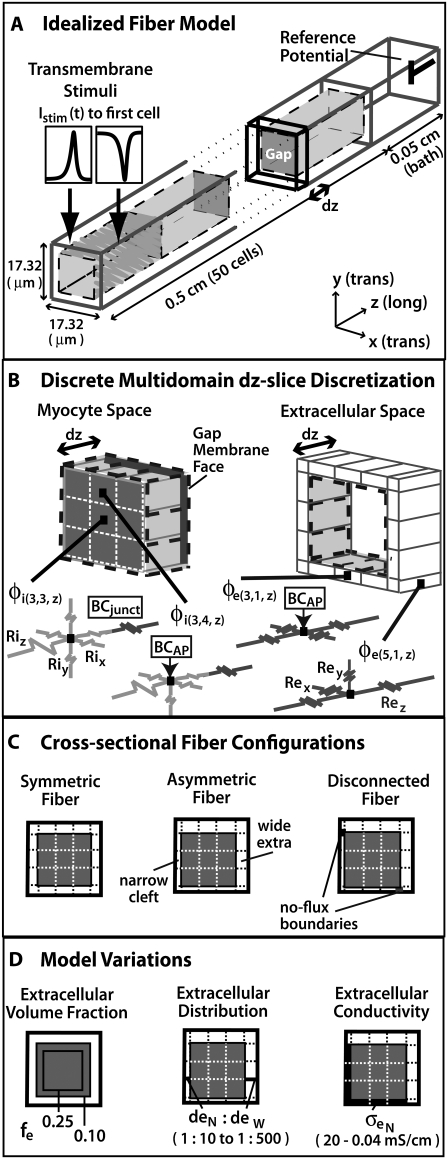
FIGURE 1.
(A) Depiction of the first two and last cells in an idealized cardiac fiber used to simulate longitudinal propagation. The model consisted of 50 rectangular cells (100 μm long) connected via intercellular junctions (Gap). Propagation was initiated via a time-variant transmembrane stimulus (shown by the two traces) applied to the entire first cell. (B) Discretization of the discrete multidomain for a single dz slice defined by nine intracellular voxels and 16 encompassing extracellular voxels. For each space, two sample resistor networks are shown. φi and φe represent the intracellular and extracellular potentials at a uniquely defined (x, y, z) coordinate within the tissue space. The boxed BCAP and BCjunct are assigned boundary conditions at locations where two domains interface. (C) Three model configurations were used that described a 1), symmetric; 2), asymmetric; and 3), asymmetric, but also discontinuous extracellular space in the tissue cross section. (D) In all model configurations, the fraction of extracellular space was varied between 0.10 and 0.25. In the asymmetric configurations, the extracellular depth was skewed such that 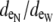 biased the distribution of extracellular space in the cross section. In an asymmetric fiber model defined by fe = 0.10 and 1:500 bias in distribution, the conductivity in the narrow cleft (
biased the distribution of extracellular space in the cross section. In an asymmetric fiber model defined by fe = 0.10 and 1:500 bias in distribution, the conductivity in the narrow cleft (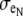 ) was isotropically increased from normal to 0.04 mS/cm.
) was isotropically increased from normal to 0.04 mS/cm.