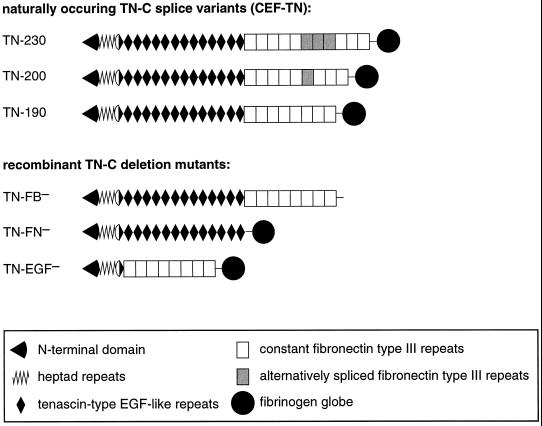
Figure 1.
Models of one subunit of naturally occuring TN-C variants as isolated from chick embryo fibroblasts (CEF-TN) and of the deletion mutants used. The subunits consist of an N-terminal part involved in the oligomerization to hexamers (N-terminal domain and heptad repeats), followed by tenascin-type EGF-like repeats, FN type III repeats, and a domain homologous to the globular part of b- and g-fibrinogen. Alternatively spliced FN type III repeats are shown as gray rectangles. Each of the deletion mutants lacks one type of domains: TN-FB− lacks the fibrinogen globe, TN-FN− lacks all FN type III repeats, and TN-EGF− lacks all EGF-like repeats. All recombinant TN-C mutants were proven to occur as hexamers and to show expected dimensions and structural features (Fischer et al., 1997).