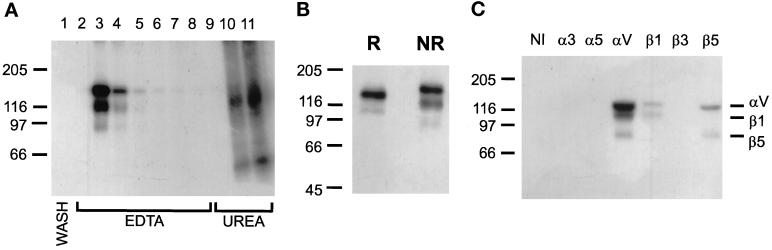
Figure 8.
Affinity purification of LAP-binding integrins in the presence of Mn2+. LAP-Sepharose affinity chromatography was done as described in Figure 8, except that buffers contained 1 mM Ca2+, Mg2+, and Mn2+. (A) Autoradiograph of fractions from the LAP affinity column, run nonreduced on a 7% gel. Lane 1: last wash fraction; lanes 2–8, elution with EDTA; lanes 9–11, elution with 6 M urea. (B) The EDTA-eluted fractions shown in B were pooled. Aliquots were electrophoresed under reducing (R) and nonreducing (NR) conditions on 7% gels and autoradiographed. (C) The same sample was divided into equal aliquots and immunoprecipitated with polyclonal antisera to cytoplasmic domains of the indicated integrin subunits or with nonimmune rabbit serum (NI). Immunoprecipitates were separated nonreduced on 7% gels and autoradiographed.