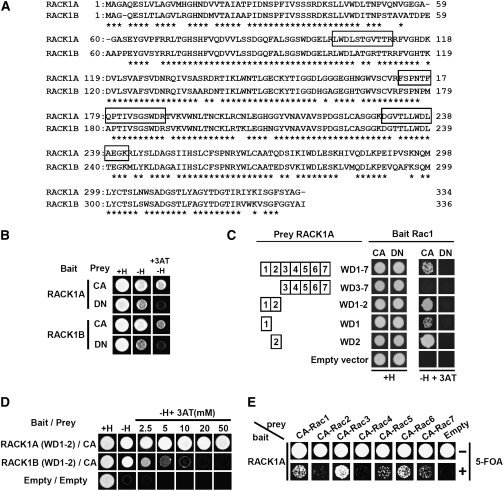
Figure 2.
Interaction of RACK1 and Rac1 in Yeast Two-Hybrid Assays.
(A) Alignment of the amino acid sequences of rice RACK1A and RACK1B. The peptide fragments identified by mass spectrometry are boxed. Asterisks represent amino acids conserved in RACK1A and RACK1B.
(B) Interaction of RACK1A and RACK1B with CA- and DN-Rac1 in yeast two-hybrid assays using a LexA-VP16 system. This two-hybrid system detects the protein–protein interaction in nuclei. Since the C-terminal Cys of Rac1, which is required for plasma membrane localization, was considered to inhibit transfer of Rac1 into nuclei, the Cys residue was exchanged with Ser. Growth on selective plates without His (−H) or without His plus 3-aminotriazole (3AT) indicates a positive interaction.
(C) Determination of specific WD40 repeats that interact with Rac1 using yeast two-hybrid assays.
(D) Difference of binding activities of RACK1A and RACK1B to Rac1.
(E) Interaction of RACK1A and the CA form of Rac family proteins in split-ubiquitin two-hybrid assays. Growth on selective plates with 5-fluroorotic acid (5-FOA) indicates a positive interaction.