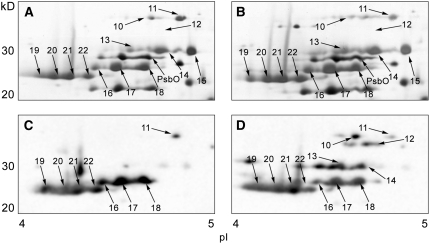
Figure 8.
Two-Dimensional Separation and Phosphorylation Analysis of Unbound LHCII Polypeptides from the State 1– and State 2–Locked Samples.
Nickel affinity chromatography flow-through from the State 1 ([A] and [C]) and State 2 ([B] and [D]) samples was subjected to 2-DE. Shown are the regions between 20 and 40 kD and pH 4.0 and 5.0 on the 2-DE gels. Protein spots were silver stained ([A] and [B]), and the numbered spots were identified by MS/MS analysis (see Supplemental Table 3 online). Phosphorylated proteins were detected using immunoblotting with an antiphosphothreonine antibody ([C] and [D]). Loaded protein amounts were approximately the same in (A) and (B), and in (C) and (D).