FIGURE 5. Oligonucleotide strand exchange.
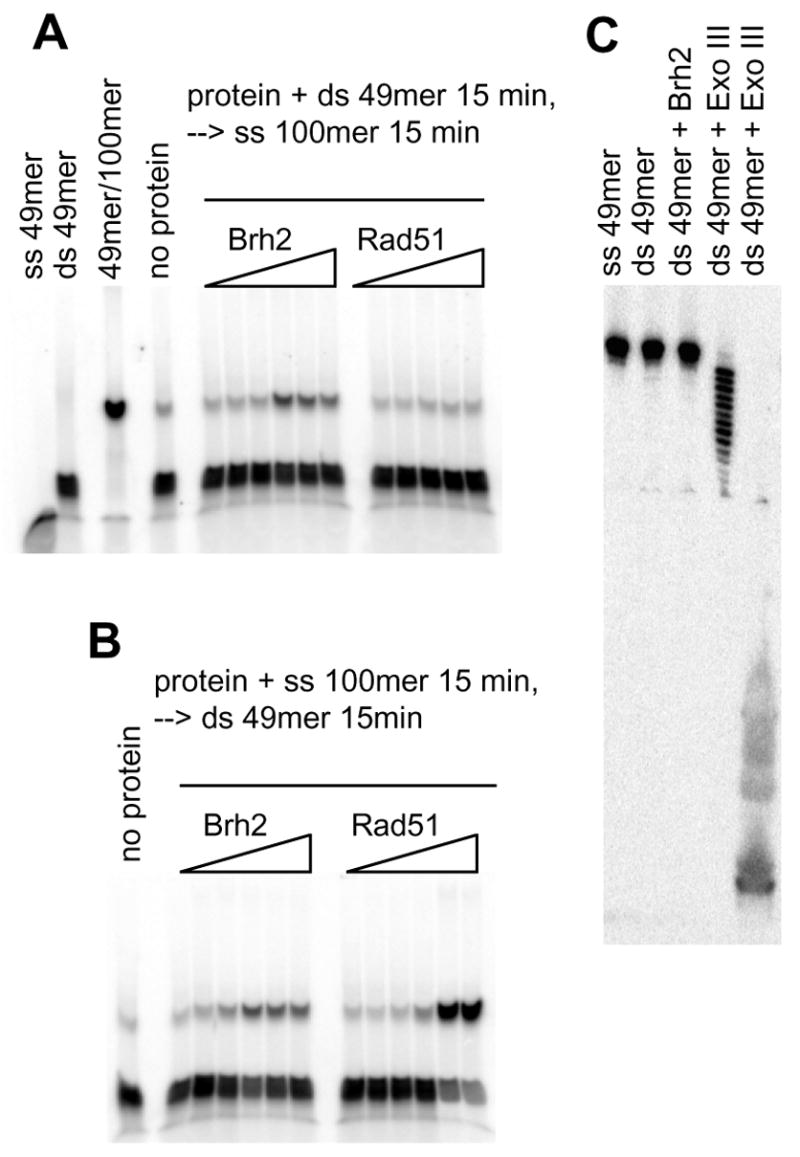
(A) Brh2 or Rad51 was preincubated with 6 nM 32P-labeled ds49mer (label on minus strand) for 15 min (4 mM CaCl2 and 2 mM ATP added for Rad51 reactions), then 60 nM ss100mer was added and incubation continued for 15 min. (B) Brh2 or Rad51 was preincubated with 60 nM ss100mer. After 15 min 6 nM 32P-labeled ds49mer was added and incubation continued for an additional 15 min. Concentrations for the samples of Brh2 in A and B were 0.05, 0.1, 0.25, 0.5. 1, and 2 μM, respectively. For Rad51, concentrations were 0.06, 0.15, 0.6, 1.5. and 3 μM in A and 0.06, 0.15, 0.3, 0.6, 1.5. and 3 μM in B. (C) Brh2 (1 μM) was incubated with 6 nM 32P-labeled ds49mer (label on minus strand) for 30 min in 25 mM Tris-HCl, pH 7.5, 20 mM KCl, 4 mM MgCl2, 1 mM DTT, 2 mM ATP. Similarly, reactions were run containing 0.004 units or 1 unit Exo III, respectively. Samples were mixed with tracking dye stop solution containing formamide and EDTA to yield final concentrations of 50% and 10 mM, respectively, heated at 50° for 5 min, then loaded on an 8% polyacrylamide gel containing 7 M urea that had been prerun for 30 min to warm the gel.
