Figure 1.
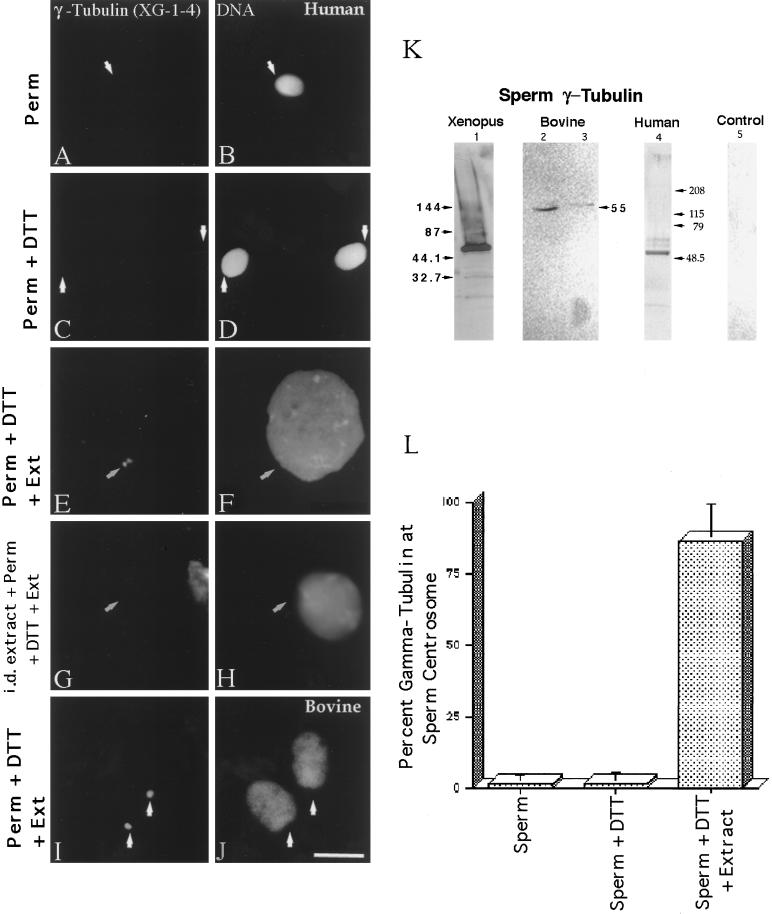
γ-Tubulin in human and bovine sperm centrosomes. More than 98% of human sperm do not immunostain with the XG-1-4 γ-tubulin antibody (A; arrow points to the sperm centrosomal region) after lysolecithin permeabilization and methanol fixation (B, DNA). Likewise, no significant increase in the detection of γ-tubulin at the human sperm centrosome is observed after 5 mM DTT priming treatment (C; arrows point to the sperm centrosomal region), although some DNA decondensation occurs in vitro (D). Very similar observations have been observed in bovine spermatozoa treated in exactly the same manner. However, both human (E and F) and bovine (I and J) spermatozoa treated with 5 mM DTT followed by CSF-arrested cell-free extract exposure demonstrated extensive DNA decondensation after 1 h (F and J) and XG-1-4 γ-tubulin immunolocalization at the sperm centrosomal regions (E and I; arrows point to the sperm centrosomal region).Immunodepletion of γ-tubulin from the CSF-arrested extracts by the XG-1-4 γ-tubulin abolishes detection of γ-tubulin at the base of permeabilized, DTT-treated human spermatozoa, demonstrating that the vast majority of γ-tubulin is maternally derived (G and H). (K) Western blot analysis of Xenopus, human, and bovine sperm demonstrates prominent bands at ∼55 kDa with the XG-1-4 antibody, indicating the presence of paternal γ-tubulin in these sperm. Lane 1, Xenopus sperm, 1.25 × 106 sperm per lane; lane 2, bovine sperm subjected to Percoll density centrifugation, at ∼2.6 × 106 sperm per lane; lane 3, washed bovine sperm without Percoll separation, at ∼2.6 × 106 sperm per lane; lane 4, human sperm subjected to Percoll density separation and labeled with XG-1-4 γ-tubulin antibody, ∼2.5 × 106 sperm per lane; lane 5, 0.5 μg of purified α- and β-tubulin, demonstrating no cross-reactivity of these tubulin superfamily members with the XG-1-4 γ-tubulin antibody. (L) Graphic representation of permeabilized human spermatozoa immunostained with γ-tubulin XG-1-4 antibody after permeabilization, DTT priming, and CSF-arrested cell-free extract. By immunofluorescence, very little paternal γ-tubulin is observed in permeabilized human sperm (left bar) or permeabilized human sperm primed by exposure to 5 mM DTT (middle bar). However, a significant increase in the detection of γ-tubulin is observed when permeabilized and primed sperm are treated with CSF-arrested cell-free extract (right bar). All images were double labeled for γ-tubulin and Hoechst DNA. (Arrows) Sperm centrosomal region as observed with phase or differential interference contrast optics. Bar in J, 10 μm.