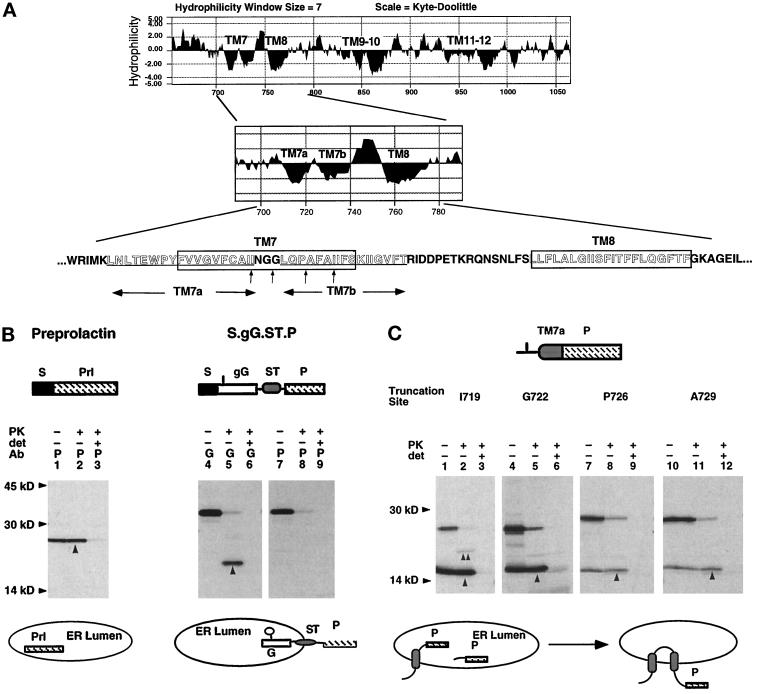
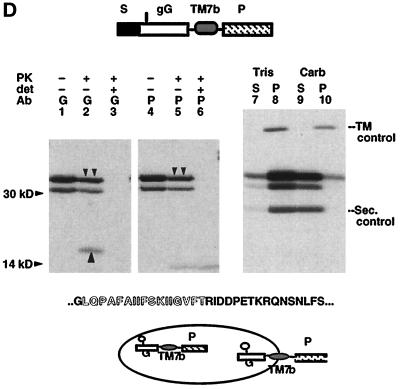
Figure 2.
Signal and ST activities of TM7a and TM7b. (A) Kyte–Doolittle hydropathy profile of the C-terminal MDR1-Pgp hydrophobic domain. Location and sequence of potential TM segments are indicated. (B) Mature XOs expressing bovine prolactin (lanes 1–3) and the TM protein S.gG.ST.P (Skach and Lingappa, 1994) (lanes 4–9) were homogenized and digested with PK in the presence and absence of detergent (det), immunoprecipitated with antiprolactin (P) or antiglobin (G) antisera, and analyzed by SDS-PAGE. Upward arrows indicate polypeptides protected from PK digestion. Topology of chains is diagrammed below the autoradiogram. The glycosylation site is indicated in the gG reporter as a vertical line. (C) Plasmids encoding TM7a fused to the P reporter at indicated residues (A, vertical arrows) were expressed in oocytes and analyzed as in B. Upward arrows (lanes 2, 5, 8, and 11) indicate chains protected from protease digestion. Topology of chains is indicated below the autoradiogram to reflect that most uncleaved chains (60–80%) pellet with membranes at neutral pH (our unpublished observations). (D) Plasmid S.gG.TM7b.P was expressed in oocytes and analyzed as in B. Downward arrows indicate full-length, PK-protected glycosylated chains (lanes 2 and 5). Upward arrow (lane 2) indicates the PK protected globin-reactive fragment. For carbonate extraction (lanes 7–10), oocyte homogenates containing S.gG.TM7b.P and TM (S.L.ST.gG.P; Skach and Lingappa, 1993) and secretory (bovine prolactin; Skach and Lingappa, 1993) control proteins were incubated in neutral buffer (Tris) or 0.1 M Na carbonate (Carb) as described in MATERIALS AND METHODS. Supernatants (S) and pellets (P) were immunoprecipitated with prolactin antisera. Topology of chains is indicated.