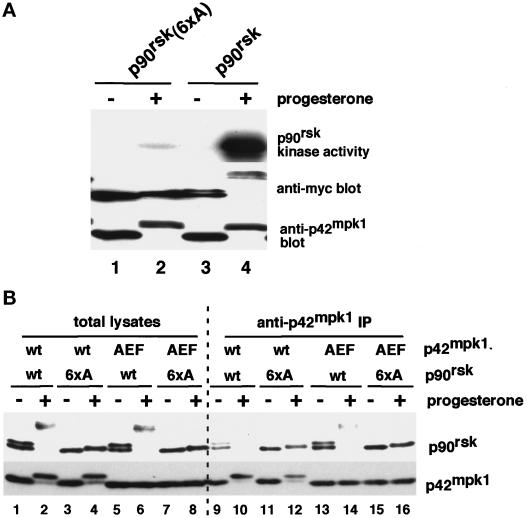
Figure 1.
Mutation to alanine of six phosphorylatable residues in p90rsk impairs its activation by progesterone and its ability to dissociate from p42mpk1. (A) Lysates were prepared from untreated or progesterone-treated oocytes expressing either myc-tagged p90rsk or p90rsk (6xA) and were analyzed by immunoblotting using anti-myc and anti-p42mpk1, as indicated. Anti-myc immunoprecipitates were prepared from the same oocyte lysates, and their associated kinase activity was assayed using GST-Myt1 as an in vitro substrate. (B) Lysates were prepared from either untreated or progesterone-treated oocytes expressing the indicated p90rsk and p42mpk1 proteins and were immunoprecipitated with anti-p42mpk1 antibodies. The total lysates (lanes 1–8) and the anti-p42mpk1 immunoprecipitates (lanes 9–16) were analyzed by immunoblotting with anti-p90rsk and anti-myc antibodies.