Abstract
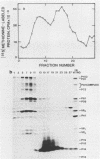
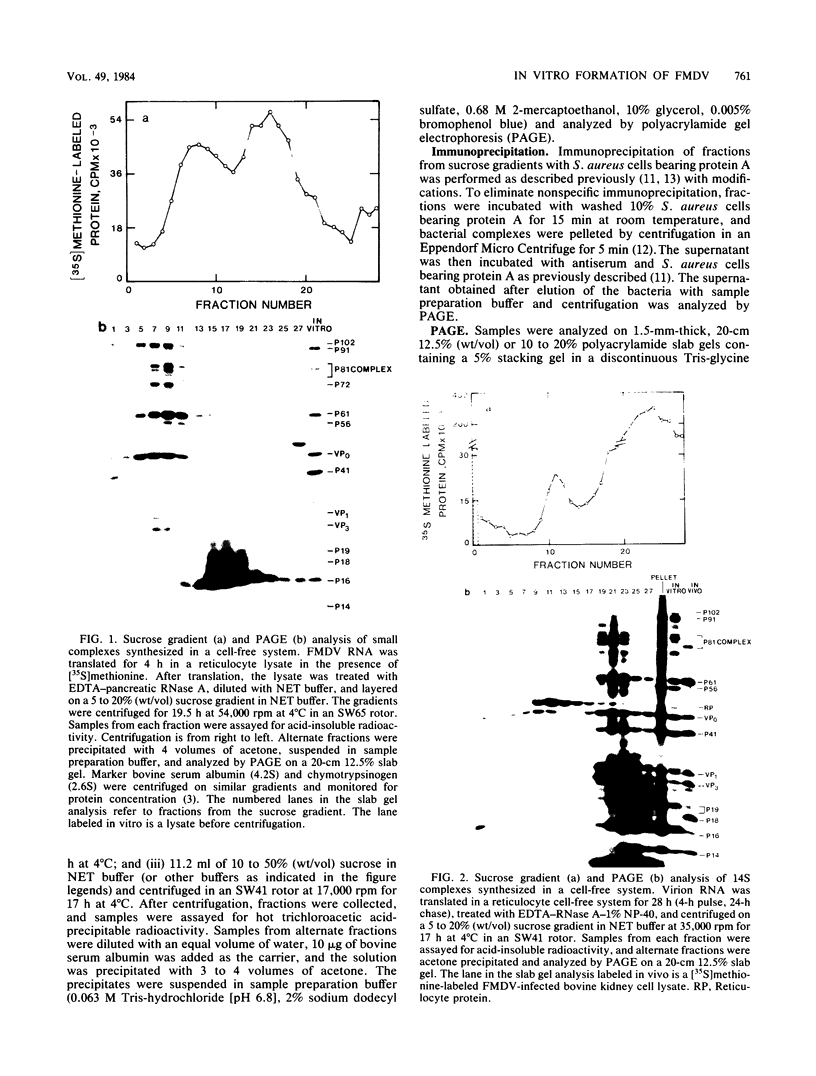
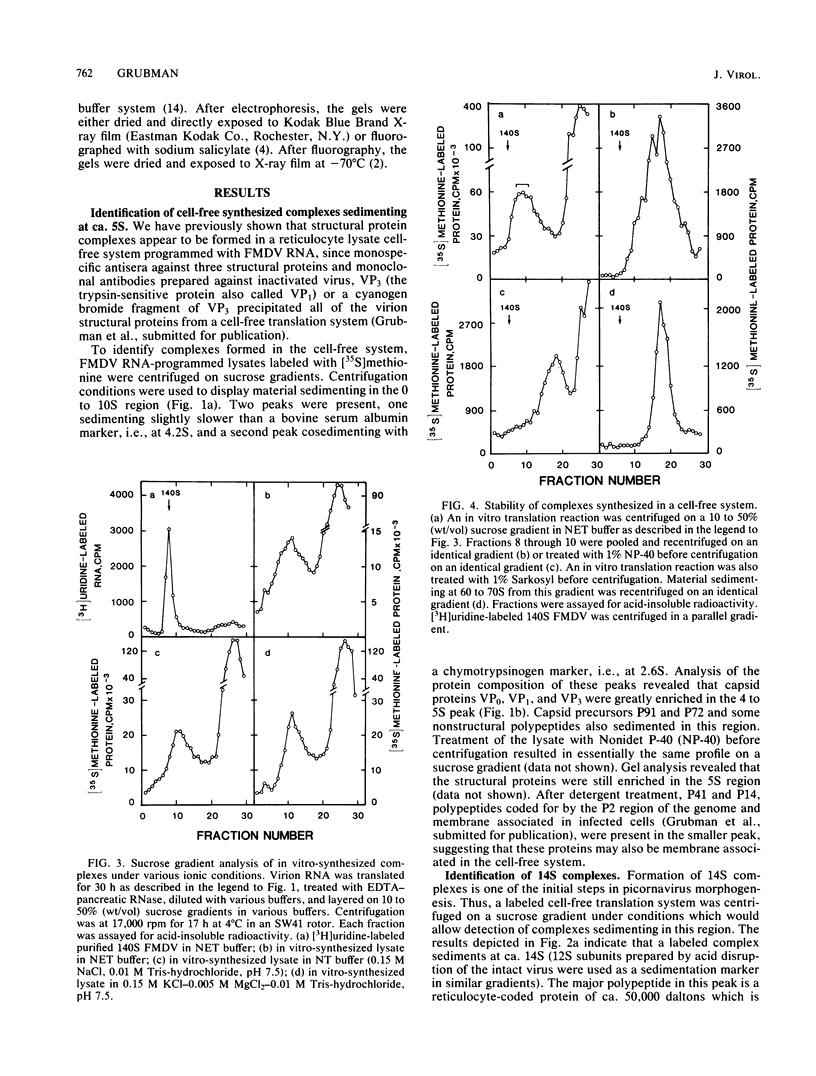
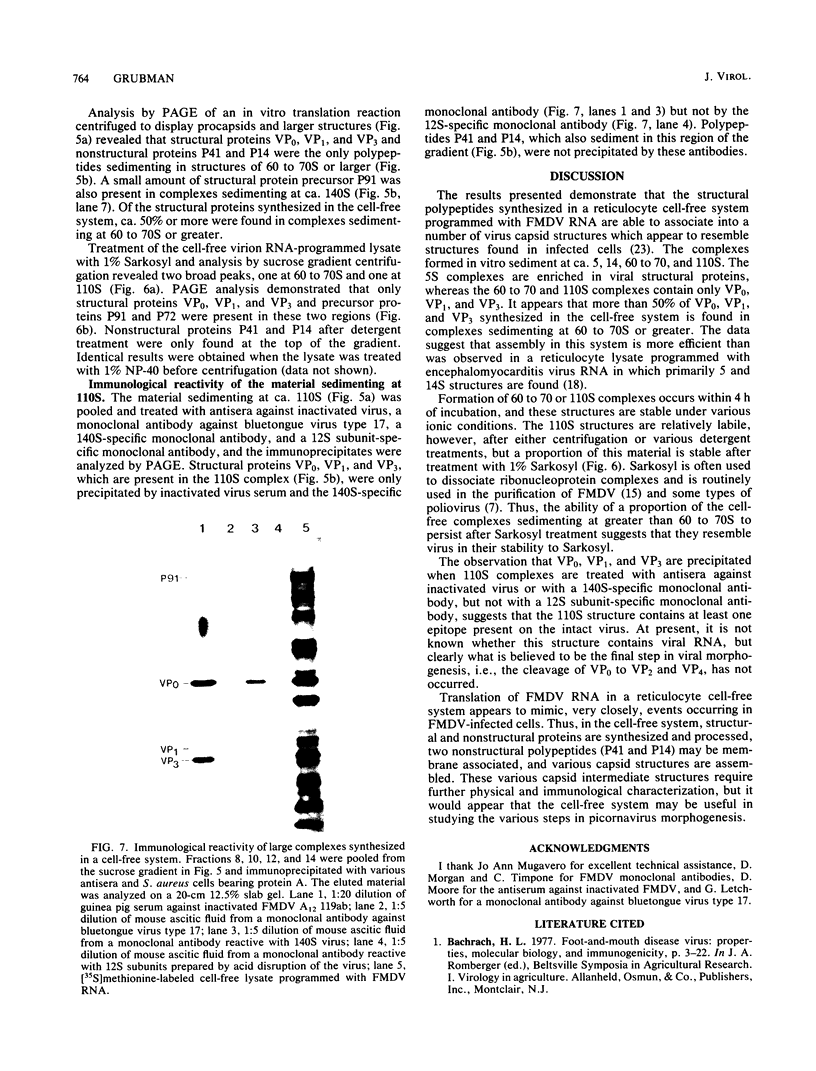
Foot-and-mouth disease virion RNA is translated efficiently and completely in a rabbit reticulocyte lysate cell-free system. Treatment of cell-free lysates with monospecific serum prepared against the individual viral structural proteins or with monoclonal antibodies prepared against the inactivated virus or against a viral structural protein precipitated all of the structural proteins, suggesting that structural protein complexes were formed in vitro. Sucrose gradient analysis of the cell-free lysate indicated that complexes sedimenting at 5, 14, 60 to 70, and ca. 110S were assembled in vitro. Structural proteins VP0, VP1, and VP3 were the major polypeptides found in these complexes. The material sedimenting at 110S, i.e., containing VP0, VP1, and VP3, was precipitated by a 140S-specific monoclonal antibody but not by a 12S subunit-specific monoclonal antibody, suggesting that this capsid structure contained at least one epitope present on the intact virus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chatterjee N. K., Polatnick J., Bachrach H. L. Cell-free translation of foot-and-mouth disease virus RNA into identifiable non-capsid and capsid proteins. J Gen Virol. 1976 Sep;32(3):383–394. doi: 10.1099/0022-1317-32-3-383. [DOI] [PubMed] [Google Scholar]
- Fernandez-Tomas C. B., Baltimore D. Morphogenesis of poliovirus. II. Demonstration of a new intermediate, the proviron. J Virol. 1973 Nov;12(5):1122–1130. doi: 10.1128/jvi.12.5.1122-1130.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszman M., Bucchini D., Girard M. Purification of the Sabin strain of poliovirus type I through treatment with sarkozyl. J Virol. 1971 May;7(5):687–689. doi: 10.1128/jvi.7.5.687-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman M. J., Bachrach H. L. Isolation of foot-and-mouth disease virus messenger RNA from membrane-bound polyribosomes and characterization of its 5' and 3' termini. Virology. 1979 Oct 30;98(2):466–470. doi: 10.1016/0042-6822(79)90570-1. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Baxt B., Bachrach H. L. Foot-and-mouth disease virion RNA: studies on the relation between the length of its 3'-poly(A) segment and infectivity. Virology. 1979 Aug;97(1):22–31. doi: 10.1016/0042-6822(79)90369-6. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Baxt B. Translation of foot-and-mouth disease virion RNA and processing of the primary cleavage products in a rabbit reticulocyte lysate. Virology. 1982 Jan 15;116(1):19–30. doi: 10.1016/0042-6822(82)90399-3. [DOI] [PubMed] [Google Scholar]
- Harris T. J., Brown F., Sangar D. V. Differential precipitation of foot and mouth disease virus proteins made in vivo and in vitro by hyperimmune and virus particle guinea pig antisera. Virology. 1981 Jul 15;112(1):91–98. doi: 10.1016/0042-6822(81)90615-2. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- La Torre J. L., Grubman M. J., Baxt B., Bachrach H. L. The structural polypeptides of aphthovirus are phosphoproteins. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7444–7447. doi: 10.1073/pnas.77.12.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGregor S. Evidence for the existence of protomers in the assembly of encephalomyocarditis virus. J Virol. 1975 May;15(5):1107–1120. doi: 10.1128/jvi.15.5.1107-1120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor S., Rueckert R. R. Picornaviral capsid assembly: similarity of rhinovirus and enterovirus precursor subunits. J Virol. 1977 Feb;21(2):548–553. doi: 10.1128/jvi.21.2.548-553.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C. In vitro synthesis and assembly of picornaviral capsid intermediate structures. J Virol. 1982 Dec;44(3):900–906. doi: 10.1128/jvi.44.3.900-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Summers D. F., Maizel J. V., Jr In vitro assembly of poliovirus-related particles. Virology. 1968 Jun;35(2):216–226. doi: 10.1016/0042-6822(68)90262-6. [DOI] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R. T., Taylor M. W. Morphogenesis of picornaviruses: characterization and assembly of bovine enterovirus subviral particles. J Gen Virol. 1976 Mar;30(3):317–328. doi: 10.1099/0022-1317-30-3-317. [DOI] [PubMed] [Google Scholar]
- Yafal A. G., Palma E. L. Morphogenesis of foot-and-mouth disease virus. I. Role of procapsids as virion Precursors. J Virol. 1979 Jun;30(3):643–649. doi: 10.1128/jvi.30.3.643-649.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]