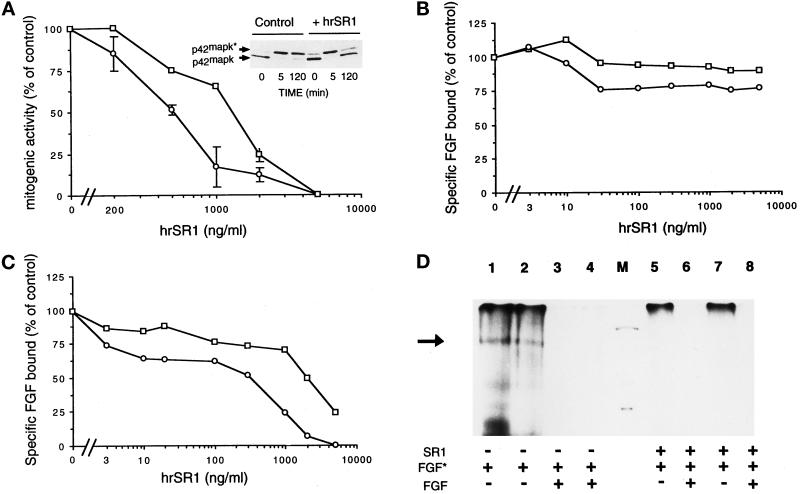
Figure 8.
In vitro effects of hrSR1 on FGF1- and FGF2-induced mitogenesis and ERK2 activation and on FGF1 and FGF2 binding in RPE cells. (A) RPE cells were incubated with varying concentration of human recombinant SR1 (hrSR1) in the presence of FGF1 (□) and of FGF2 (○) at 10 ng/ml. Mitogenic activity was measured by the incorporation of tritiated thymidine. The percentage of inhibition of activity is plotted as a function of the hrSR1 concentration. (A, top panel) ERK2 phosphorylation was detected by its characteristic retardation of electrophoretic mobility. The phosphorylated (ERK2*) and unphosphorylated (ERK2) forms of MAP kinase are indicated. (B and C) RPE cells were incubated with 125I-FGF1 (□) and 125I-FGF2 (○) at 5 ng/ml in the presence of various concentrations of hrSR1. The amount of 125I-FGF bound to high-affinity receptors (C) was measured in Triton X-100 cell extracts after cells were washed with 2 M NaCl. The radioactivity in the 2 M NaCl washes was 125I-FGF bound to the FGF low-affinity binding sites (B). Nonspecific binding of 125I-FGF to RPE cells was determined in the presence of an excess of unlabeled FGF (200-fold). No error bar is given for points, because the errors were smaller than the symbols. (D) Cross-linking of 125I-FGF1 (lanes 2, 4, 7, and 8) and 125I-FGF2 (lanes 1, 3, 5, and 6) to RPE cells. Cells were incubated with 5 ng/ml iodinated FGF (FGF*) in the presence of 700 ng/ml hrSR1 (lanes 5–8). Checks of the specificity of iodinated FGF binding in the presence of hrSR1: cell incubation with an excess of unlabeled FGF1 (lanes 4 and 8) and unlabeled FGF2 (lanes 3 and 6). Cross- linking was performed as described in MATERIALS AND METHODS. A specific band was detected at 170 kDa (black arrow). M, molecular mass markers (200, 92, and 69 kDa). In all assays, comparable results were obtained in three independent experiments.