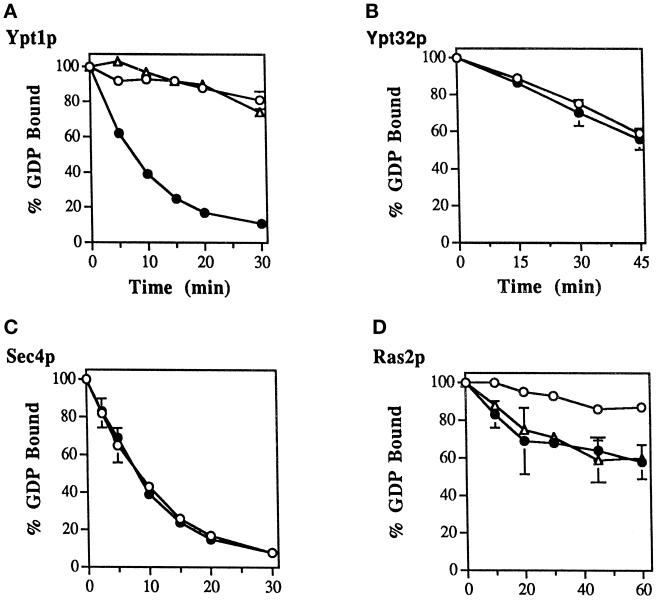
Figure 4.
The Ypt1p-GEF activity present in the Det-P100 (Triton X-100– or n-octylglucoside–extracted) fraction does not act on Ypt32p or Sec4p and is different from the exchange factor for Ras2p. Ten picomoles of Ypt1p-[3H]GDP (A), Ypt32p-[3H]GDP (B), Sec4p-[3H]GDP (C), or Ras2p-[3H]GDP (D) were incubated in the presence of the Det-P100 fraction (closed circles; 5 mg/ml, except 2 mg/ml Ypt32p) or BSA (open circles), and the stimulated and intrinsic rates of [3H]GDP release were determined by sampling at the times indicated. Including the Det-P100 fraction at 1 mg/ml resulted in ∼6-fold stimulation of GDP release from Ypt1p (our unpublished observations), but no stimulation was observed for Ypt32p at concentrations of Det-P100 up to 2 mg/ml. Including 5 mg/ml of Det-P100 fraction resulted in ∼12-fold stimulation of GDP-release from Ypt1p, no stimulation for Sec4, and approximately ∼4- to 6-fold stimulation for Ras2p. The stimulated GDP release by Ypt1p can be completely inhibited by Ypt1-D124N dominant-mutant protein (1.5 μM). However, the GEF activity for Ras2p present in this fraction is not affected by this Ypt1 mutant protein (A and D; open triangles). The results represent the averages of two experiments with duplicates for each time point. Error bars represent SEM.