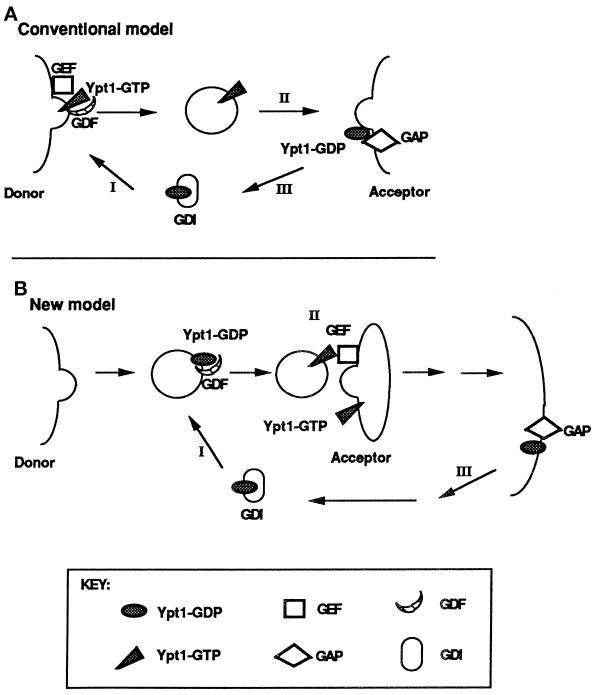
Figure 9.
Two models for the role of nucleotide cycling and factors that regulate it in Ypt/Rab-mediated vesicular transport, using Ypt1p as an example. (A) Conventional model (Goud and McCaffrey, 1991; Novick and Brennwald, 1993). In addition to GEFs and GAPs, two other factors that influence nucleotide cycling of Ypt/Rab proteins are GDI and GDI-dissociation factor (GDF). GDI is implicated in the recycling of Ypt/Rab proteins between membranes (Araki et al. 1990; Soldati et al., 1993), and GDFs are thought to function as receptors or chaperones for Ypt/Rab proteins (Dirac-Svejstrup et al., 1997). Step I, recruitment of Ypt1p-GDP to the donor membrane by GDF and nucleotide exchange by GEF to yield Ypt1p-GTP are shown; Ypt1p-GTP is present on forming secretory vesicles. Step II, GTP hydrolysis is required or is coupled with fusion of secretory vesicles with the acceptor compartment. Step III, GDI recycles Ypt1p-GDP back to the donor membrane. (B) New model, based on this article and our previous work (Jones et al., 1995; Richardson et al., 1998). Step I, Ypt1p-GDP is recruited to the vesicle (or the donor membrane) by GDF. Step II, nucleotide exchange by GEF is coupled to vesicle fusion with the acceptor compartment. Step III, GTP hydrolysis occurs late in the pathway to generate Ypt1p-GDP, and GDI recycles Ypt1p-GDP for the next cycle. The important features that distinguish this model from the conventional model are the major role suggested for nucleotide exchange and the factor that mediates it (GEF), the minor role of GTP hydrolysis and GAP not in vesicle fusion but in Ypt1p recycling, and the suggested localization of these regulators. If GAP localizes to the plasma membrane, as shown here, it might have a role in GDI-mediated Ypt/Rab protein recycling (which is not required for Ypt1p function). In ypt1-Q67L mutant cells, when GTP hydrolysis is defective, Ypt1p-GTP might be recycled via a GDI-independent mechanism. If GAP localizes to the ER, it might be there to stimulate GTP hydrolysis by Ypt1p to allow better interaction with the GEF in the next cycle (see DISCUSSION).