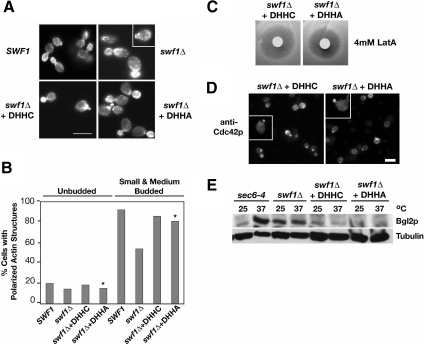
Figure 8.
Actin organization, Cdc42p localization, and Bgl2p-marked secretion were independent of the Swf1p DHHC motif. (A) Micrographs of rhodamine-phalloidin-stained, log phase, haploid, wild-type SWF1 cells (KKY1064), mutant swf1Δcells (KKY 1063), and swf1Δ cells with CEN plasmid that contained wild-type (+DHHC; KKY1086) or mutant SWF1 (+DHHA; KKY1087). All strains were grown at 25°C. SWF1 and swf1Δ stains were grown on rich medium and swf1Δ strains containing a plasmid were grown in SC-URA. Bar, 5 μm. (B) Comparison of the number of cells with polarized cortical actin cytoskeleton, at different cell cycle phases. The cortical actin cytoskeleton was scored as polarized when cortical actin patches were distributed to one pole of unbudded cells or were distributed to the bud in small- and medium-budded cells. All scored cells had a single nucleus as visualized with DAPI. n > 200 cells scored for each morphological class. The cells quantified were from the same experiment shown in A. Asterisks highlight the strain that lacked the DHHC motif. (C) Halo assay with the same strains used in A plated on SC-Ura and incubated at 25°C for 2 d. Filter disks were impregnated with 4 mM LatA. (D) Indirect immunofluorescence micrographs of log-phase swf1Δ cells expressing Swf1-DHHCp (KKY1086; left) or Swf1-DHHAp (KKY1087; right), grown at 25°C in SC-Ura, labeled with anti-Cdc42p and goat anti-rabbbit-Alexa 568 antibodies. Bar, 5 μm. (E) Immunoblot shows from left to right the internal level of secretory marker Bgl2p in log phase sec6-4ts (NY17), swf1Δ (KKY1063), swf1Δ + DHHC (KKY1086), and swf1Δ + DHHA (KKY 1087) cells, cultured for 90 min, at 25 or 37°C in rich and SC-Ura medium. To demonstrate equivalent loading, the same blot was probed with an antibody against β-tubulin.