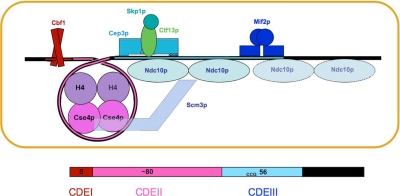
Figure 6.
Diagram summarizing binding data in this article, in previous work on CBF3–centromere interactions (Espelin et al., 1997), and in the literature. CBF3 comprises a Skp1p:Ctf13p heterodimer associated closely with a Cep3p dimer; addition of one or two dimers of Ndc10p generates “core” and “extended” complexes, respectively. The Cse4p nucleosome contains Scm3p, Cse4p, and H4 in 1:1:1 proportion, but it seems to lack H2a and H2b (Mizuguchi et al., 2007). An interaction of Scm3p and Ndc10p has been proposed to be the mechanism by which CBF3 recruits the Cse4p nucleosome (Camahort et al., 2007). We discuss in the text whether adding a second Ndc10p dimer, to form an extended complex, is compatible with recruitment of Mif2p; the possibility, that the two are mutually exclusive is suggested by a white hatching across the second Ndc10p dimer. The guide at the bottom of the figure relates the spatial organization diagrammed in the upper part to the sequence and organization of centromeric DNA in yeast.