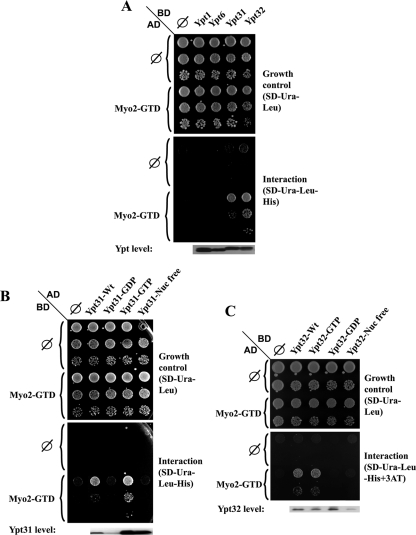
Figure 1.
Yeast two-hybrid interaction of Ypt31/32 GTPases and Myo2-GTD. (A) Ypt specificity: Ypt32 and Ypt31, but not Ypt1 and Ypt6, interact with the Myo2-GTD in the yeast two-hybrid mating assay. Yeast MATa (NSY468) cells expressing Myo2-GTD from the pACT2 (GAL4-AD, LEU2) vector were mated with MATα (NSY752) cells expressing the indicated Ypts from pAS1 (GAL4-BD, TRP1) plasmids: Ypt1, Ypt6, Ypt31, and Ypt32. Diploids were selected on SD-Trp-Leu medium. Growth control is shown on SD-Trp-Leu (top), and interaction is shown on SD-Trp-Leu-His (bottom). Cells were plated in 10-fold serial dilutions from top to bottom and incubated at 30°C. Empty vector (∅) for both plasmids are shown as negative controls. Immunoblot analysis shows similar Ypt expression levels by using anti-Gal4 binding domain antibody (bottom). (B) Nucleotide-bound form specificity of the Ypt31–Myo2-GTD interaction: Myo2-GTD interacts specifically with the wild-type and the GTP-bound form of Ypt31, but not with its GDP-bound and nucleotide-free form in the yeast two-hybrid assay. Yeast MATα cells expressing Myo2-GTD from the pGBDU-C2 (GAL4-BD, URA3) vector were mated with MATa cells expressing the various nucleotide-bound forms of Ypt31 from pACT2 (GAL4-AD, LEU2) plasmids: wild type, Q72L (GTP), S27D (GDP), and D129N (nucleotide free). Plating, negative controls, and immunoblot analysis were done as described in A, except for using anti-Gal4 activation domain. Growth control is shown on SD-Ura-Leu (top), and interaction is shown on SD-Ura-Leu-His (bottom). (C) Nucleotide-bound form specificity of the Ypt32–Myo2-GTD interaction: Myo2-GTD interacts specifically with the wild-type and the GTP-bound form of Ypt32, but not with its GDP-bound and nucleotide-free form in the yeast two-hybrid assay. Yeast cells were transformed with two plasmids: Myo2-GTD was expressed from the pACT (GAL4-AD, LEU2) vector and the indicated nucleotide-bound forms of Ypt32 were expressed from pGBDU-C2 (GAL4-BD, URA3) plasmids—wild type, Q72L (GTP), S27D (GDP), and D129N (nucleotide free). Growth control is shown on SD-Ura-Leu (top), and interaction is shown on SD-TUra-Leu-His + 0.1 mM 3AT (bottom). Plating, negative controls and immunoblot analysis were done as described in B, except cells were plated in fivefold dilutions. Results shown in this figure represent at least two independent experiments.