Figure 1. PA-PhoQ is repressed by divalent cation but does not recognize CAMP.
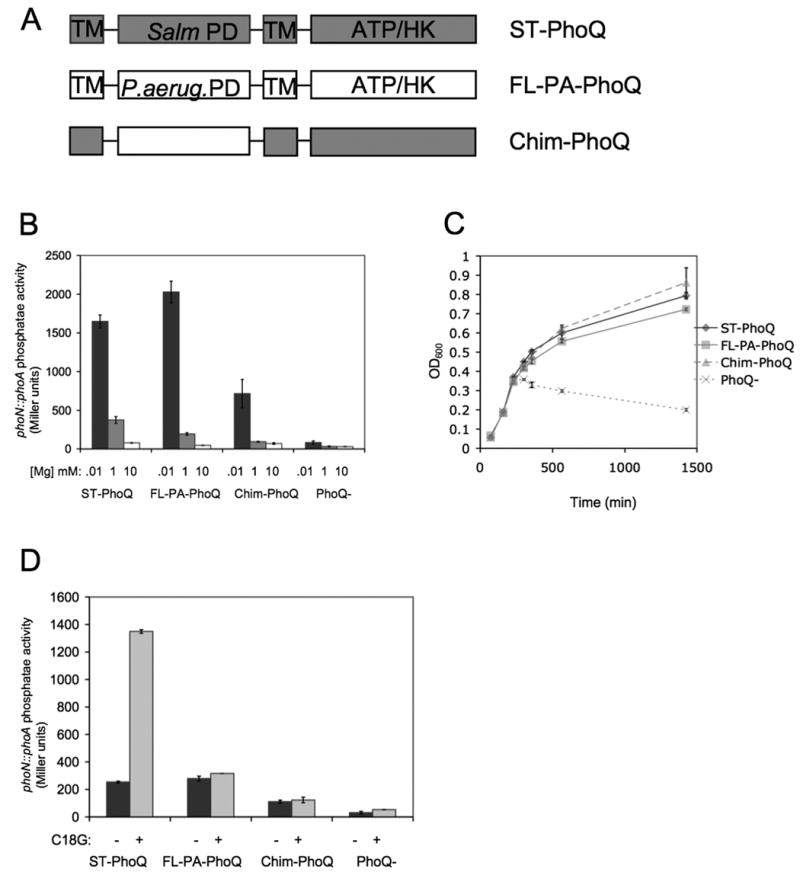
(A) A schematic of the domain organization of the PhoQ constructs used in this study. The PhoQ periplasmic domain (PD) is flanked by two transmembrane regions (TM) and contains a cytoplasmic ATP-binding domain with catalytic histidine kinase activity (ATP/HK). Chim-PhoQ consists of full-length ST-PhoQ except that the PD has been replaced with that of PA-PhoQ.
(B) PhoP-dependent gene activation decreases with increasing concentration of Mg2+ for all PhoQ constructs tested. A reporter fusion between PhoP-dependent acid phosphatase (PhoN) and PhoA was used to measure activation, and PhoQ constructs are expressed chromosomally. Cultures were grown in N minimal medium containing 10 μM (black bars), 1 mM (gray bars), or 10 mM (white bars) MgCl2.
(C) PA-PhoQ constructs can complement a PhoQ-null strain for growth in low divalent cation medium. Strains were grown in N minimal medium supplemented with 10 μM MgCl2 and growth was measured by OD600 over time.
(D) PA-PhoQ cannot activate PhoP-dependent gene activation in response to CAMP. Cultures were grown in N minimal medium supplemented with 1 mM MgCl2. Where indicated, C18G was added at 5 μg/ml. All graphed values are mean ± standard deviation of cultures grown in duplicate and are representative of at least three independent trials.
