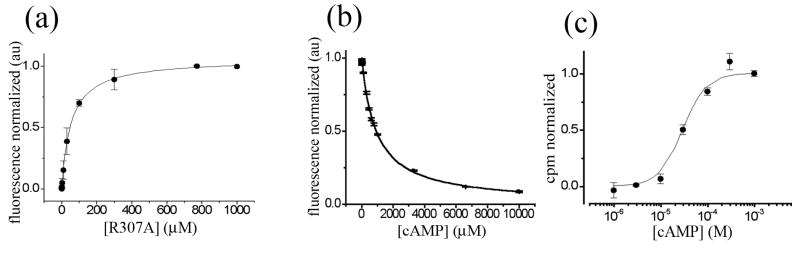
Figure 4. Binding and activation of R307A.
a) Fluorescence enhancement upon R307A CNB domain binding to 8-NBD cAMP. Purified protein was titrated (0 to 1mM) against 2 μM fluorescent ligand and a binding affinity of 45.1 ± 5.8 μM was determined. b) Competition by unlabelled cAMP. 50 μM of pure R307A was incubated with 150 μM 8-NBD cAMP and subsequently competed with unlabelled cAMP (0 to 10 mM). A binding affinity of 205.1 ± 10.4 μM was determined. c) Concentration dependence of nucleotide-mediated uptake as measured with the 86Rb+ flux assay. Uptake at 90 min was normalized to basal uptake in the absence of nucleotide (0.0) and the maximal uptake (1.0). K1/2 Activation of 30 ± 4.9 μM and a Hill coefficient (n) of 1.7 ± 0.4 was determined.