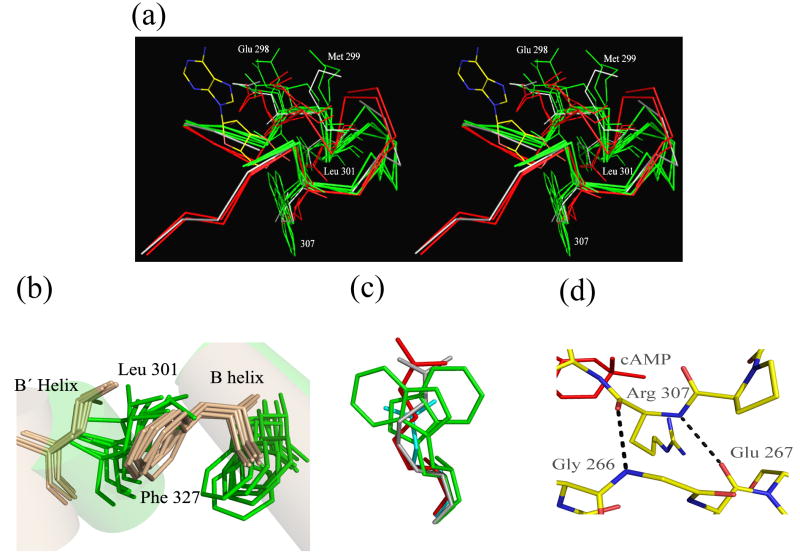
Figure 9. Features of the Binding Pocket.
a) Overlay of the binding pockets (residues 295-312) of two bound: wild type-cAMP (red), R307E (red) and 5 apo: R348A (gray) R307W (green) structures. Glu 298 and Met 299, both highly mobile, are highlighted. The side chains of Leu 301 and position 307 (various mutants) as well as cAMP are shown for reference. b) The positions of Leu 301 and Phe 327 in the bound (tan) and apo structures (green) showing clear differences in the position of the side chains between the two states but not amongst the structures in the individual states. c) The position of the Arg 307 side chain is invariant regardless of state or mutation. Side chain position shown: R307 from cAMP-bound wild type (red), R307 from the R348A apo structure (gray), Glu 307 from the R307E bound structure (cyan) and two different Trp 307s from the R307W apo structure (green). d) Stabilization of position 307 by interactions between its main chain and residues of a parallel loop. Hydrogen bonds between the backbones at Arg 307, Gly 266 and Glu 267 from the cAMP-bound wild type structure are shown.