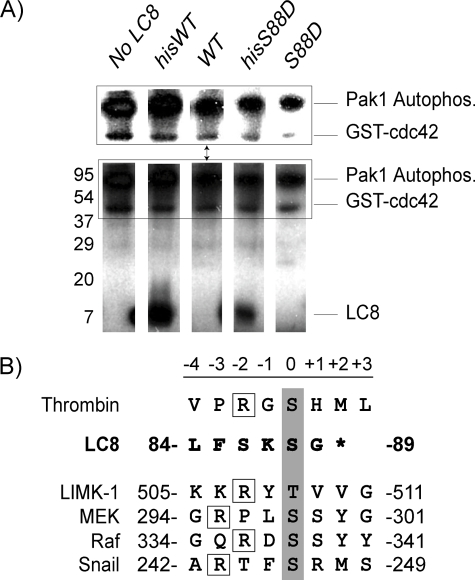
FIGURE 8.
Pak1 does not phosphorylate LC8 in vitro. A, in vitro Pak1 kinase assay. Activated Pak1 kinase assays were performed using [32P]ATP, separated by SDS-PAGE, and detected by autoradiography. Individual lanes are shown for each reaction. Pak1 phosphorylates His-tagged WT-LC8 and the phosphomimic His-tagged LC8-S88D. These proteins contain a thrombin cleavage site C-terminal to the His tag and N-terminal to LC8. The same LC8 proteins in which the His tag was removed are not phosphorylated by activated Pak1. The inset above shows phosphorylation of Pak1 and GST-Cdc42 at different contrast settings. GST-Cdc42, which also contains a thrombin site C-terminal to the GST tag, is also phosphorylated in these assays. These experiments were repeated three times. B, the thrombin cleavage site contains a Pak1 phosphorylation consensus sequence. Residues that are phosphorylated by Pak1 are highlighted in gray. Boxed are the upstream arginine residues important for Pak1 target phosphorylation. As shown, LC8 does not contain the Pak1 consensus phosphorylation sequence.