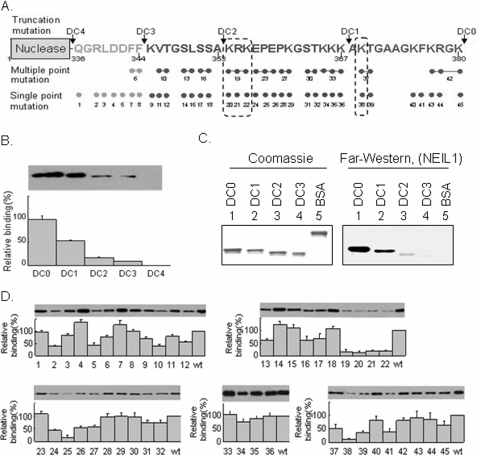
FIGURE 2.
Mapping of the interacting domain of FEN-1 and residues for NEIL1. A, design of the scanning mutations in the C-terminal region of FEN-1. WT (DC0), nested deletions, DC1, DC2, DC3, DC4, and single- and multiple-point mutations used in this study are represented. The arrows indicate the truncation sites. B, mapping the interaction domain of FEN-1 with NEIL1 by pull-down assay. NEIL1 was immobilized on Sepharose beads and incubated with WT and C-terminal deletion mutants of FEN-1. The bound proteins were separated on a 4–15% gradient SDS-PAGE and detected using FEN-1 antibody. C, far Western analysis of membrane-bound WT and deletion mutants of FEN-1 with NEIL1 in solution, as in Fig. 1C. D, identification of interacting residues in FEN-1 with NEIL1 by pull-down assay. Upper panels, immunoblots with FEN-1 antibody; lower panels, binding of single- and multiple-point mutants of FEN-1 with NEIL1, relative to WT FEN-1.