Scheme 3.
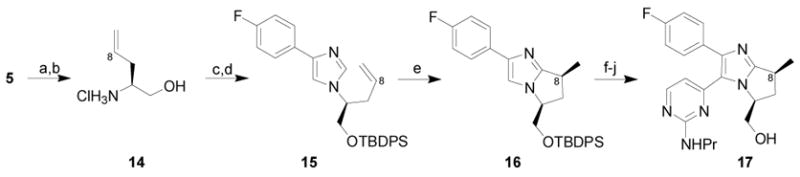
Synthesis of C8 Methyl Bicyclo Bis-Arylimidazole Derivative
Conditions: (a) allylmagnesium bromide, CH2Cl2, −78 °C to rt, 92% (single diastereomer); (b) 4N HCl, CH3OH, 94%; (c) 4-fluorophenyl tosylmethyl isonitrile,3 glyoxylic acid, K2CO3, DMF; (d) TBDPSCl, iPr2NEt, DMAP, CH2Cl2, 78% (over 2 steps); (e) [RhCl(coe)2]2, PCy3, MgBr2, toluene, 180 °C, 52%, 92% ee; (f) Br2, CH2Cl2, −78 °C, 80%; (g) 2-methylthio-4-trimethylstannylpyrimidine,6 Pd2(dba)3·CHCl3, PPh3, LiCl, CuI, dioxane, 170 °C, 84%; (h) OXONE®, THF, H2O; (i) propylamine, 91% (over 2 steps); (j) TBAF, THF, 99%.
