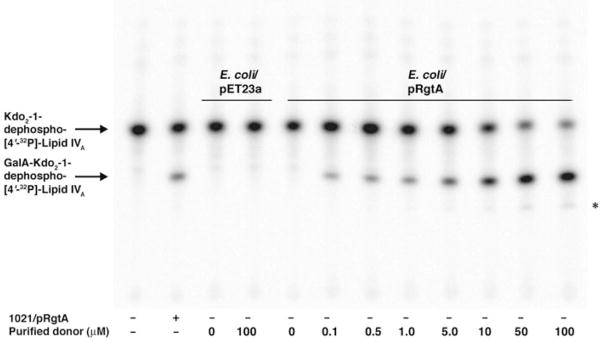
FIGURE 7. In vitro reconstitution of RgtA activity with purified dodecaprenyl phosphate-GalA.
RgtA was assayed using 2.5 μM of Kdo2-1-dephospho-[4′-32P]lipid IVA as the acceptor substrate under standard conditions for 5 min with 0.25 mg/ml membrane protein. The dodecaprenyl phosphate-GalA was dissolved in the same assay buffer, except that the Triton X-100 concentration was increased to 0.2% to dissolve the purified lipid. Membranes from E. coli NovaBlue (DE3) harboring pRgtA or the vector pET23a were used as indicated. The purified donor substrate was added in increasing concentrations, ranging from 0.1 to 100 μM. The reconstituted E. coli system was run alongside S. meliloti 1021/pRgtA control membranes.