Abstract
We investigated the hypothesis that the enhanced antigen-presenting function of IL-10 deficient dendritic cells (DCs) is related to specific immunoregulatory cytoskeletal molecules expressed when exposed to antigens. We analyzed the role of a prominent cytoskeletal protein, LEK1, in the immunoregulation of DC functions; specifically cytokine secretion, co-stimulatory molecule expression, and T cell activation against Chlamydia. Targeted knockdown of LEK1 expression using specific anti-sense oligonucleotides resulted in the rapid maturation of Chlamydia-exposed DC as measured by FACS analysis of key activation markers (i.e., CD14, CD40, CD54, CD80, CD86, CD197, CD205 and MHC II). The secretion of mostly Th1 cytokines and chemokines (IL-1a, IL-9, IL-12, MIP-1a, and GM-CSF but not IL-4 and IL-10) was also enhanced by blocking of LEK1. The function of LEK1 in DC regulation involves cytoskeletal changes, since the dynamics of expression of vimentin and actin, key proteins of the cellular cytoskeleton, were altered after exposure of LEK1 knockdown DC to Chlamydia. Furthermore, targeted inhibition of LEK1 expression resulted in the enhancement of the immunostimulatory capacity of DCs for T cell activation against Chlamydia. Thus, LEK1 knockdown DCs activated immune T cells at least 10-fold over untreated DCs. These results suggest that the effect of IL-10 deficiency is mediated through LEK1-related events that lead to rapid maturation of DCs and acquisition of the capacity to activate an elevated T cell response. Targeted modulation of LEK1 expression provides a novel strategy for augmenting the immunostimulatory function of DCs for inducing an effective immunity against pathogens.
Keywords: Chlamydia, dendritic cells, immunomodulation, immunity, vaccine
Introduction
Efficacious vaccines are needed against the widespread diseases of several intracellular microbial pathogens, including the oculo-genital diseases caused by Chlamydia trachomatis whose control requires a prominent T cell immunity. A potentially efficacious chlamydial vaccine should induce an adequate T cell response and the accessory antibodies (1-3). DCs are primary antigen-presenting cells (APCs) for inducing high levels of T cell immunity (4, 5), and have been established to play a major role in controlling chlamydial infection in both animals and humans (3, 6). DCs reside in an immature state in most non-lymphoid sentinel tissue sites, and upon encounter of an antigen, they undergo maturation which promotes their migration to the draining secondary lymphoid organs, where they initiate immune responses (7). During maturation, DCs strongly up-regulate MHC antigens, and costimulatory molecules such as CD40, CD80 and CD86, which are crucial for effective T cell activation. We previously reported that IL-10 deficiency rendered DCs to become highly potent APCs for activating a high level of predominantly T helper type 1 (Th1) response against Chlamydia in vitro and in vivo (8). It was hypothesized that IL-10 deficient DCs expressed specific immunostimulatory molecules that promote the ability to rapidly activate a high level of specific T cell response.
Subsequent proteomic analysis to identify immunoregulatory molecules associated with the potency of IL-10 deficient established that IL-10 deficiency caused an early maturation and activation of DCs after exposure to Chlamydia. DC activation was marked by expression of high levels of activation markers, and consequently an enhanced ability to process and present antigens for a rapid and robust T cell activation (8). Furthermore, a group of cytoskeletal proteins, namely actin, vimentin and LEK1 were identified to be differentially expressed in IL-10 deficient DCs that were exposed to Chlamydia but their role in antigen handling and T cell activation was unclear. The elucidation of the direct or indirect role of such molecules in antigen handling by DCs can lead to strategies to modulate their expression in vaccine delivery against Chlamydia. There is increasing evidence that the cytoskeleton is involved in DC uptake and handling of antigens, migration to lymphoid tissues and maturation, and leading to effective presentation for T cell activation. The functional changes occurring during DC maturation are associated with dynamic and specialized alterations in the configuration of the actin cytoskeleton, which involve Rho GTPases, Rac, Cdc42, Rho and the Wiskott-Aldrich Syndrome Protein (WASp) (7, 9, 10). Thus, appropriate and timed cytoskeletal reorganization may control the effector functions of DCs, including antigen handling and T cell activation (7, 11, 12). In this study, we analyze in greater detail one of the cytoskeletal molecules, LEK1, identified by proteomics to be differentially expressed by IL-10 deficient DCs, as compared to wild-type (WT) DCs. LEK1 is a member of the LEK family of proteins and known to play a role in cell differentiation and microtubule function relating to cytoskeletal organization and cell shape (13, 14). With an estimated molecular mass of 200kDa, LEK1 undergoes posttranslational cleavage that produces two peptides. The C-terminal fragment (nucLEK1) localizes to the nucleus and function in the regulation of cell division and differentiation; however, the N-terminal peptide (cytLEK1) remains in the cytoplasm and plays a role in the regulation of the cytoskeleton (15). The cytLEK1 consists of a spectrin repeat region and numerous leucine zippers that regulate pocket protein activity during developmental processes (13, 15). Since a spectrin repeat serves as a cytoskeletal and signal transduction docking region and leucine zippers participate in protein-protein interactions, we propose that LEK1 is involved in the cytoskeletal events that are related to antigen acquisition, APC maturation, processing and presentation. The results from our study provide evidence for the involvement of LEK1 in regulating DC maturation and APC function for Th1 activation.
Materials and Methods
Animals
Female IL-10−/− (IL-10 knockout, IL-10KO) and the control IL-10+/+ mice on a C57BL/6J background (The Jackson's Laboratory, Bar Harbor, MA) were fed with food and water ad libitum and maintained in laminar flow racks under pathogen-free conditions of 12 h light and 12 h darkness. The animal use protocols described in this proposal have been approved by the IACUC of Morehouse School of Medicine and CDC.
DC isolation and culture
DCs were isolated from the bone marrows of WT and IL-10KO mice by the standard method and differentiated by in vitro culture with IL-4 and GM-CSF, as previously described (8). DCs were characterized as loosely adherent mononuclear cells and determined by FACS analysis to express high levels of MHC class II, CD54 (ICAM-1), and CD11c. After five days in culture, DCs were washed and used in the experiments as described.
Proteomic analysis
DCs from WT and IL-10KO mice were pulsed with chlamydial elementary bodies (EBs) derived from Chlamydia trachomatis agent of mouse pneumonitis (MoPn) for 2 or 8 h and lysed with a rehydration buffer containing 8 M urea, 10 mM DTT, 2 M CHAPS, 0.2% bio-Lyte (Bio-Rad Laboratories, Hercules, CA). FOCUS-Protease Arrest and FOCUS-Protease Nuclease (EMD Chemicals, Inc., Gibbstown, NJ) were added to a final concentration of10μg/ml. The amount of proteins was determined by using the Bio-Rad RC DC protein assay kit (Bio-Rad Laboratories). 200ug of each sample was separated by 2-dimensional gel electrophoresis (2-DE). Selected spots corresponding to specific proteins on the SDS-PAGE were analyzed by MALDI-TOF-TOF, as previously described (8).
Morpholino-based Antisense Oligomer
The antisense morpholino oligomers of LEK1 were supplied by Gene Tools, LLC (Corrallis, OR). The sequence of the antisense oligomer was 5′-AGCTCCTCACAGAACCTGGCTCCG-3′ (LEK1-ASMor-50), which is an effective inhibitor of LEK1 expression (13). The standard control oligomer (Gene Tools, LLC) had an inert sequence (5′-CCTCTTACCTCAGTTACAATTTATA-3′) with no cellular gene target and no detectable biological activity. The oligomers were prepared according to the supplier's protocol and administered to cultured cells using the suggested the Endo-Poter delivery system. The final concentration of LEK1 mophilino oligomers was 10μM per 106 cells and the treatment did not cause any phenotypic changes on the cells as verified by microscopy.
FACS analysis
WT DCs were cultured in 24-well plates and treated with either standard control oligo or LEK1-ASMor-50 for 48 h. Non-treated or oligo-treated cells were then pulsed with UV-inactivated MoPn EBs for 1 h and the supernatants were collected for cytokines and chemokines analysis. Cells were harvested and washed with FACS buffer at 4 °C, and stained with FITC- or phycoerythrin-conjugated antibodies against CD11c, CD14, CD40, CD54, CD80, CD86, CD197, CD205 and MHC II. The effect of LEK1 knockdown on the expression of these molecules on chlamydial-pulsed DC was measured on a FACScan flow cytometer (BD Biosciences, San Jose, Calif) using the CELLQuest software. Cytokines and chemokines were measured by the Luminex assay (Bio-Rad Laboratories).
Western blotting
WT DCs were cultured in 24-well plate and treated with either standard control oligo or LEK1-ASMor-50 for 48 h. The cells were then pulsed with UV-inactivated MoPn EBs and the cells were harvested at different time points, and total protein was extracted using a SDS sample buffer. 20ug protein was subjected to SDS-PAGE and transferred onto nitrocellulose membrane. Proteins (actin and vimentin) were probed by appropriate primary and secondary antibodies at concentrations recommended by the manufacturer. The band intensity was determined by a PhosphorImager (Amersham Biosciences, Piscataway, NJ).
Confocal microscopy
WT and LEK1-knockdown DCs were harvested, adjusted to a concentration of 1X106/ml and pulsed with MoPn EBs that were stained with CarboxyFluoroscein Succinimidyl Ester (CFSE) for 60 min at 37°C. The cells were washed three times and fixed for 15 min in 1% paraformaldehyde at room temperature. For cell surface staining, DCs, were treated with a phycoerythrin-conjugated goat anti-mouse CD11c antibody (BD Biosciences, San Jose, CA), washed, and stained with LysoTracker® Yellow-HCK-123 (Molecular Probes, Carlsbad, CA) for 30 min on ice for late endosomes (16). Confocal microscopy was performed with Leica TCS SP5 confocal microscope (Leica Co. Mannheim, Germany). Triple-color images were acquired using a sequential acquisition mode to avoid cross-excitation, according to the supplier's protocol, and photo-prints were processed in Adobe PhotoShop.
T cell stimulation in vitro
Spleen cells from chlamydial-infected WT mice were enriched for T cells by the nylon wool adherence method and analyzed for CD3+ cells by FACS. 1 × 105 WT DCs or LEK1 knockdown DCs were co-cultured with 2 × 105 purified T cells in the presence or absence of chlamydial Ag in 96-well tissue culture plates for 5 days, as previously described (8). The supernatants were collected and assayed for IL-4, IL-10, IL-17, and IFN-γ expression by multiplex array according to the supplier's instructions. The concentration of the cytokine in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (±SD) of triplicate cultures for each experiment. The results were derived from three independent experiments.
Results
Expression pattern of LEK1 in Chlamydia-pulsed DCs
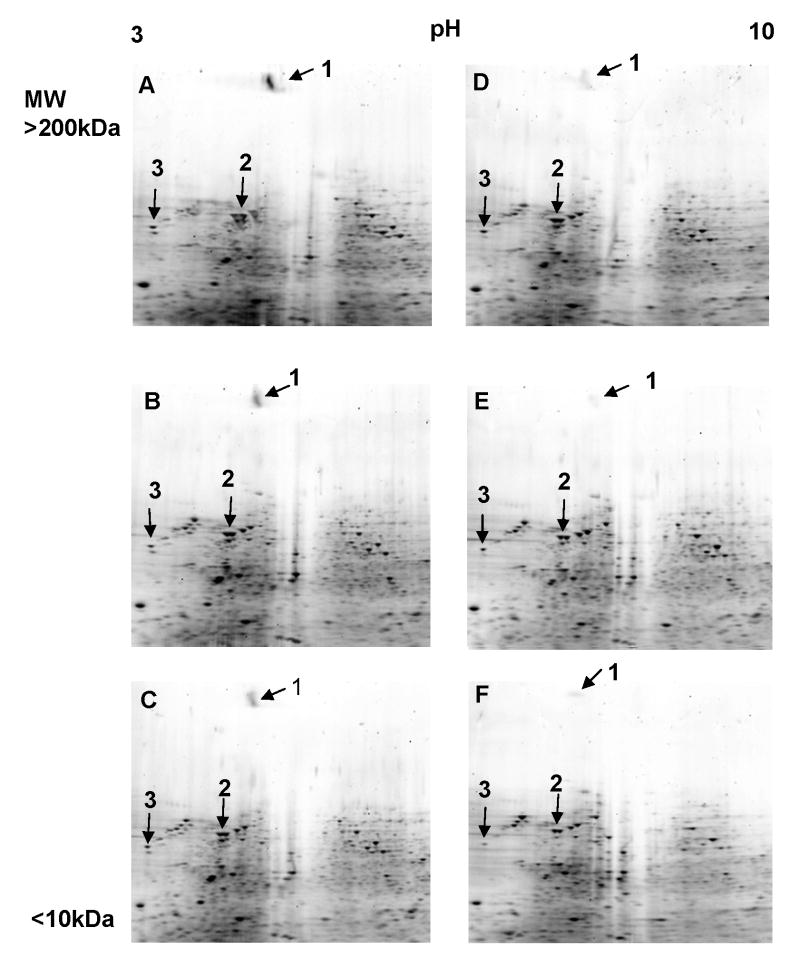
To identify and characterize specific proteins that may play a role in the enhanced T cell activating capacity of IL-10 deficient DCs, combined 2-DE and MALD-TOF proteomics were used to identify differentially expressed proteins when WT and IL-10KO DCs were exposed to Chlamydia in culture. Figure 1 shows that LEK1 is one of the differentially expressed proteins identified on a 2-D gel, with an estimated molecular mass of 200kD. LEK1 was down-regulated in Chlamydia-pulsed IL-10KO DCs at 2 and 8h of exposure. Since DCs undergo maturation by expression of activation markers during these time periods following exposure to chlamydiae (8), the results would suggest that the regulation of LEK1 is a maturation-dependent process. Thus, as DC matured, the expression of LEK1 decreases. It was interesting that certain other cytoskeletal proteins, specifically vimentin and actin, were also similarly and differentially expressed as LEK1. The prominence of LEK1 among the differentially expressed proteins led us to further characterize its role in DC functions in immune regulation.
Figure 1.
Pattern of expression of LEK1 in Chlamydia-pulsed, wild-type (WT) and IL-10KO DCs. MoPn-pulsed DCs (MOI 1:5) were analyzed with 2-DE and MALD-TOF proteomics to identify differentially expressed proteins, as described in the Materials and Methods section. Figures 1A and 1D = control WT DCs and IL-10 KO DCs, respectively, that were not pulsed with MoPn. Figures 1B and 1E = WT DCs and IL-10 KO DCs respectively, that were pulsed with MoPn for 2 h. Figures 1C and 1F = WT DCs and IL-10 KO DCs respectively, that were pulsed with MoPn for 8 h. The differentially expressed proteins that were selected for MALDI-TOF identification are shown in numbers. Spot 1, 2, and 3 were identified as LEK1, vimentin, and actin, respectively.
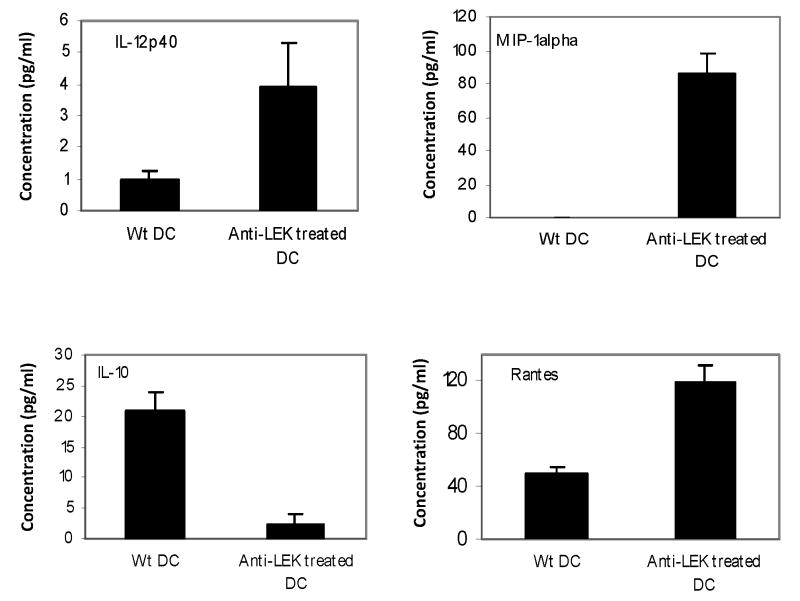
Effect of targeted inhibition of LEK1 expression on DC maturation and expression of cytokines
To assess the immunologic function of LEK-1 in DC activation when exposed to chlamydial antigens, we analyzed the effect of targeted inhibition of its expression on DC maturation and expression of cytokines. Figure 2 revealed that blocking of LEK1 expression with antisense oligos enhances the production of IL-12p40, RANTES, MIP-1 alpha, MIP-1 beta, and MCP-1 but inhibits IL-10 expression by Chlamydia-pulsed WT DCs. The control oligomer with a scrambled sequence, as described in the Materials and Methods section, did not show any detectable biological activity.
Figure 2.
Effect of targeted inhibition of LEK1 expression on DC expression of cytokines. WT DC and LEK1 knockdown DC were pulsed with MoPn (MOI 1:5) for 1 h and the supernatants were analyzed for cytokines expression (indicated in the figure), using Luminex technology, as described in the Materials and Methods section. Results represent the means of 3 independent experiments. The control oligomer with a scrambled sequence, as described in the Materials and Methods section, did not show any detectable biological activity.
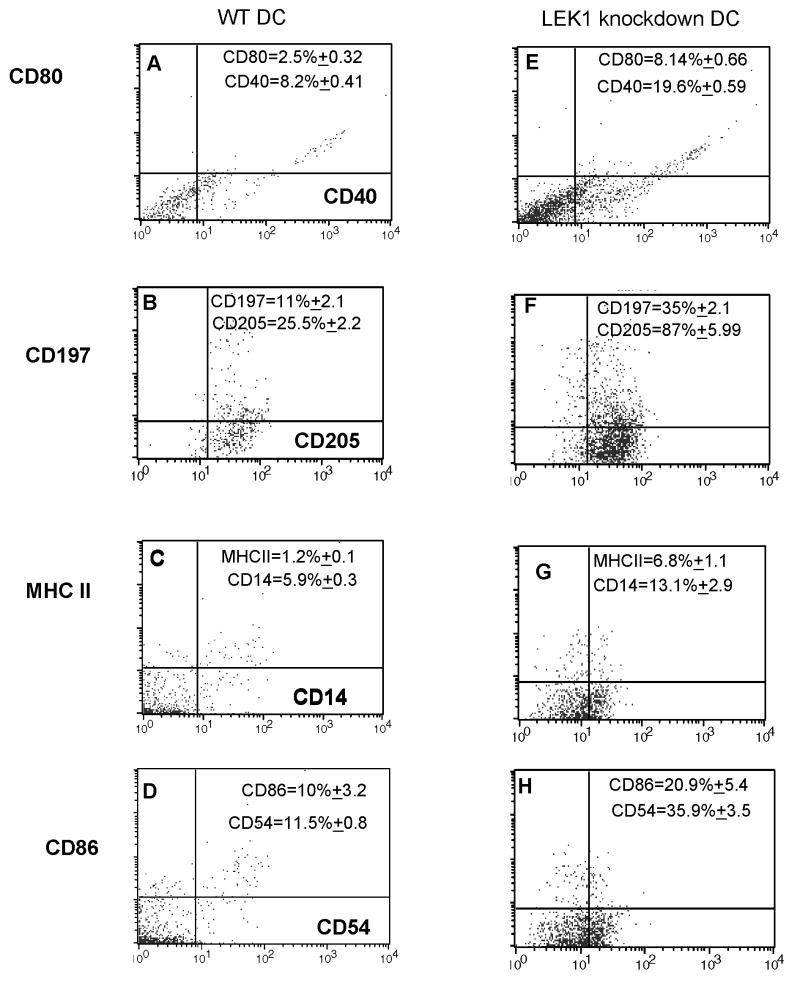
Since these conditions facilitate rapid DC maturation and enhancement of T cell activation, we also measured the expression of key activation markers on anti-sense treated WT DCs that were exposed to Chlamydia. Figure 3 shows the results from FACS analysis of CD14, CD40, CD54, CD80, CD86, CD197, CD205, and MHC II expression by anti-LEK1-treated WT DCs that were exposed to MoPn, indicating an increase in the expression of these molecules after LEK1 knockdown. Thus result indicated that LEK1 knockdown results in the rapid maturation and activation of DCs when exposed to Chlamydia, suggesting that LEK1 is directly or indirectly involved in regulating DC function.
Figure 3.
Effect of targeted inhibition of LEK1 expression on DC expression of maturation and co-stimulatory molecules. WT and LEK1 knockdown DCs were pulsed with MoPn (MOI 1:5) for 1 h and the cells were stained with FITC- (x-axis; FL1-H) and PE- (y-axis; FL2-H) conjugated mAbs against the indicated surface markers and analyzed by flow cytometry after gating for DCs according to scatter criteria. The percentage of cells bearing a single (FITC or PE) or double (FITC and PE) stains in a total of 10,000 were obtained. The results are representative plots for 3 independent experiments. The indicated mean percentages (and SD) of markers were derived from those 3 experiments. Figures 1 A, B, C, and D are WT DCs; while E, F, G, and H are LEK1 knockdown DCs. The control oligomer with a scrambled sequence, as described in the Materials and Methods section, did not show any detectable biological activity.
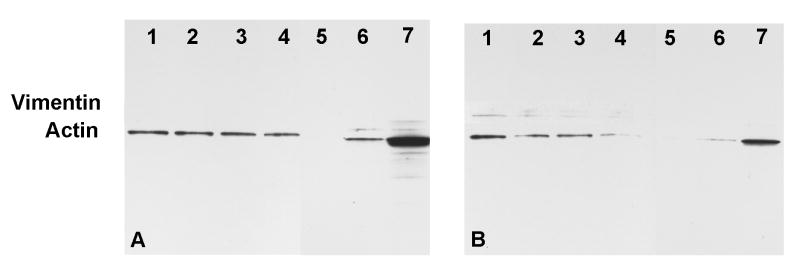
To investigate whether the function of LEK1 in DC regulation involves cytoskeletal changes, we analyzed the dynamics of expression of vimentin and actin, key proteins of the cellular cytoskeleton. Western blotting analysis of the kinetics of expression of actin and vimentin after exposure of LEK1 knockdown DCs to Chlamydia revealed that both proteins were significantly decreased, as compared to untreated DCs (Figure 4). The results indicated that cellular cytoskeletal reorganization involving tubulin (actin) and intermediate filaments (vimentin) may partially be regulated by LEK1 action.
Figure 4.
Western blot analysis of the effect of LEK1 knockdown on actin and vimentin expression in DCs. WT and LEK1 knockdown DCs were pulsed with MoPn (MOI 1:5) and analyzed for actin and vimentin expression as described in the Materials and Methods section. Figure 4A = WT DCs; 4B = LEK-1 knock down DCs. Lane 1 = non-pulsed DC; Lanes 2, 3, 4, 5, 6, and 7 were MoPn-pulsed DC for 15′, 30′ 1h, 2h, 3h, and 4h respectively. Results are representative of 2 independent experiments.
Evidence for LEK1 regulation of antigen-processing events in DCs
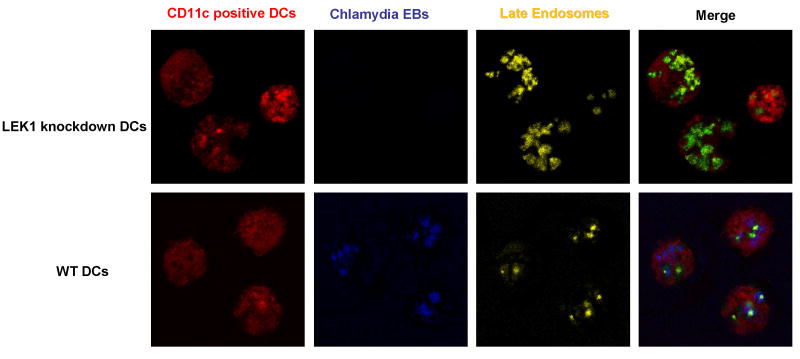
The late endosome (also called the MHC class II compartment, MIIC) is the composite lysosomal-Golgi intracellular vesicle for peptide loading, where processed antigens interact with MHC II molecules from the trans-Golgi, and represents a critical stage in APC function (17, 18). We hypothesized that the cytoskeletal functions regulated by LEK1 are related to late endosome formation and function, such that the presence of LEK1 would affect the rate of formation and accumulation of the vesicles. To test this hypothesis, we compared the rate of late endosome formation and accumulation in WT and LEK1 knockdown DCs that were pulsed with CSFE-labeled chamydial EBs. Figure 5 revealed that LEK1 down results high accumulation of LysoTracker® Yellow-HCK-123-stained late endosomes. In addition, unlike WT DCs, CSFE-labeled EBs could not be co-localized with the late endosomes in LEK1 knockdown, suggesting a rapid disappearance of the EBs in the absence of LEK1. The data may indicate that the cytoskeletal events regulated by LEK1 are related to the rate of antigen processing and late endosome formation.
Figure 5.
Role of LEK1 in late endosome activity in chlamydial-pulsed DCs. WT and LEK1 knockdown DCs were pulsed with CSFE-labeled chamydial EBs and treated with LysoTracker® Yellow-HCK-123 (Molecular Probes) to stain for late endosomes, as described in the Materials and Methods section (16). Confocal microscopy was performed and triple-color images (red = CD11c+ DCs; blue =CSFE-labeled chlamydial EBs; yellow = late endosomes; and merging of colors co-localizes endosomes and EBs in DCs) were acquired according to the supplier's protocol.Merged images (green) show a high level of late endosomes and absence of EBs in LEK1 knockdown DC; however, WT DCs showed numerous EBs and limited level of late endomal activity.
Effect of targeted inhibition of LEK1 expression in DCs on T cell activation against Chlamydia
We hypothesized that the rapid maturation and activation of Chlamydia-pulsed DCs due to LEK1 knockdown will result in an enhancement of the immunostimulatory capacity of the DCs for T cell activation against Chlamydia. To test this hypothesis, anti-sense-treated and MoPn-pulsed DCs were used to re-stimulate T cells from MoPn-infected mice in a recall culture system in vitro. The results in Figure 6 reveals that anti-sense LEK1 treatment enhanced immunostimulatory action of DCs, such they activated immune T cells at least 10-fold over untreated DCs. The increase in IFN-gamma and IL-17 in these cultures would suggest that both the protective CD4, Th1, and Th17 cells are activated against Chlamydia at the same time. Interestingly, the expression of the Th1-related chemokines and cytokines such as MIP-1a, GMCSF, IL-9 and IL-12 was boosted in these cultures as well (data not shown). The results corroborate the data shown in Figure 2 that the suppression of LEK1-related events in the absence of IL-10 leads to rapid maturation of DCs and acquisition of the capacity to activate an elevated T cell response.
Figure 6.
Effect of LEK1 knockdown on antigen-presentation for T cell activation by DCs. WT and LEK1 knockdown DCs were pulsed with UV-inactivated MoPn (MOI 1:5) for 1 h and co-cultured with nylon wool-purified T cells from immune animals in the presence or absence of chlamydial antigen, as previously described (21). At the end of the incubation period, the supernatants were collected and assayed for IFN-γ content by a quantitative sandwich ELISA, as described in the Materials and Methods section. The results are presented as Fold Increase (Response in LEk1 knockdown DC cultures divided by Response in control cultures). The response data were analyzed by calculating the mean values from 3 each of LEK1 knockdown and control experiments. The control oligomer with a scrambled sequence, as described in the Materials and Methods section, did not show any detectable biological activity.
Discussion
Chlamydia-pulsed IL-10 deficient DCs constitute an effective cellular vaccine capable of inducing a protective immune response against chlamydial genital infection in mice. The likely mechanism by which IL-10 deficiency enhances the APC function of DCs remains unclear although the process appear to be related to cellular maturation and increased in the immunostimulatory ability of the DCs. We have proposed that IL-10 deficient DCs acquire increased immunostimulatory properties due at least partly to certain immunoregulatory molecules that affect antigen uptake, processing and presentation to T cells (8). The identification and characterization of the immunostimulatory molecules that regulate the ability of IL-10 deficient DCs to activate a high level of protective immunity will facilitate vaccine design against certain intracellular pathogens and tumors.
Using a proteomic approach to analyze WT and IL-10KO DCs to identify differentially expressed molecules, we have identified LEK1, a prominent cytoskeletal protein whose expression is markedly suppressed when DCs acquired highly effective immunostimulatory function due to IL-10 deficiency. LEK1 is a member of the LEK family of proteins and known to play a role in cell differentiation and microtubule function (13, 14). LEK1 consists of a spectrin repeat region and numerous leucine zippers. A spectrin repeat serves as a cytoskeletal and signal transduction docking region and leucine zippers participate in protein-protein interactions. The disruption of LEK1 resulted in alteration of microtubule organization and cellular shape (13, 14). This would suggest that LEK1 is involved in cytoskeletal reorganization during the maturation of DCs, which may affect antigen processing and presentation. In fact, it has been suggested that appropriate and timely actin rearrangement is vital for DC effector function, including TLR-mediated activation of antigen uptake (11) and DC-T cell interaction during antigen presentation for T cell activation (12). Our studies revealed that IL-10 deficiency resulted in LEK1 suppression and acquisition of an enhanced T cell activation by DCs. Similarly, targeted LEK1 knock-down in DCs also resulted in the suppression of IL-10 secretion, and the increased immunostimulatory capacity of the DCs. Thus, IL-10 may suppress DC maturation and APC function for T cell activation through LEK1 action in the cytosketal network. This conclusion is supported by results showing that LEK1 is involved in the regulation of expression of key proteins of the cellular cytoskeleton of DCs, vimentin and actin that are involved in various cellular functions (18) (Figure 4). Other corroborating reports have suggested that DC function is associated with dynamic and specialized alterations in the configuration of the actin cytoskeleton (7), and these processes involve the GTPases Cdc42, Rac and Rho (9). In addition, the high rate of late endosome formation in LEK1 knockdown DCs would suggest that the absence of LEK1 leads to an enhanced antigen processing and presentation function. This would support the hypothesis that LEK1 regulates antigen-processing events in DCs, and indicated that the cytoskeletal events regulated by LEK1 favor late endosome formation in the absence of LEK1. These events may include the vesicular fusion relating to the formation of late endosomes, as well as the intracellular trafficking of MHC molecules to the peptide loading sites. Facilitation of these processes will likely culminate in an enhanced DC function associated with antigen processing and presentation for T activation.
As a direct confirmation of the role of LEK1 in the regulation of DC function for T cell activation, we have provided compelling results that Chlamydia-pulsed, LEK1 knockdown DCs activated T cells by approximately 10-fold compared to control DCs (Figure 6). Ongoing experiments are assessing the effect of targeted inhibition of LEK1 expression in DCs on the induction of protective immunity against Chlamydia in vivo. Our prediction is that Chlamydia-pulsed, LEK1 knockdown DCs will also induce an enhanced T cell activation in vivo, as compared to WT DCs, which would result in a greater level of protective immunity against Chlamydia in vivo.
Mechanistically, LEK1 appears to negatively regulate cytoskeletal reorganization that affects DC maturation, signaling events that promote cytokine and costimulatory molecule expression, efficient processing and presentation of antigen for T cell activation. This effect appears to involve the regulation of expression of actin and vimentin which are components of the microtubule and microfilament network of the cell, as well as late endosome formation for peptide loading on MHC molecules. Thus, molecules that regulate the cytoskeleton are important modulators of the APC functions of DCs. Biochemically, the presence of IL-10 or LEK1 either delays or limits the cytoskeletal reorganization processes that culminate in efficient antigen handling and T cell activation by DCs. The known involvement of LEK1 in cell division and vesicular fusion associated with the endocytic pathway (14) would suggest that its presence causes cytoskeletal reorganization that promotes cell division and maintenance of DCs in the immature stage. Thus, the absence of LEK1 will lead to cytoskeletal events or reorganization that favor DC maturation as exhibited by IL-10 deficient or targeted LEK1 anti-sense-treated DCs. We propose that the action of LEK1 is exerted at multiple levels that include post-internalization events, such as endocytic maturation, antigen processing and presentation since certain defects in cytoskeletal functions have been associated with suppression of these events (7, 10, 19, 20). Besides, the pharmacological manipulation of LEK1 expression in vivo may lead to an enhanced immune response to potential vaccines against Chlamydia, other intracellular microbial pathogens and tumors that are controlled by robust T cell immunity.
Acknowledgments
We appreciate the technical assistance of Dr. Xuebiao Yao and Andrew Shaw.
Grant Support: This work was supported by PHS grants (AI41231, GM 08248 and RR03034) from the National Institutes of Health and the Centers for Disease Control and Prevention (CDC).
Abbreviations
- IL-1KO
interleukin 10 knockout
- APCs
antigen-presenting cells
- Th1
T helper type 1
- Th2
T helper type 2
- MALDI-TOF
matrix-assisted laser desorption/ionization-time-of-flight
- 2-DE
two dimensional gel electrophoresis
References
- 1.Hafner L, McNeilly C. Vaccines for Chlamydia infections of the female genital tract. Future Microbiol. 2008;3:67–77. doi: 10.2217/17460913.3.1.67. [DOI] [PubMed] [Google Scholar]
- 2.Roan N, Starnbach M. Immune-mediated control of Chlamydia infection. Cell Microbiol. 2008;10:9–19. doi: 10.1111/j.1462-5822.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 3.Brunham R, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 4.S RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37:S53–S60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 5.hhBanchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D, Yang X, Lu H, Zhong G, Brunham RC. Immunity to Chlamydia trachomatis mouse pneumonitis induced by vaccination with live organisms correlates with early granulocyte-macrophage colony-stimulating factor and interleukin-12 production nad with dendritic cell-like maturation. Infect Immun. 1999;67:1606–1613. doi: 10.1128/iai.67.4.1606-1613.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns S, Thrasher AJ. Dendritic cells: the bare bones of immunity. Curr Biology. 2004;14:R 965–R967. doi: 10.1016/j.cub.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 8.He Q, Moore TT, Eko FO, Lyn D, Ananaba GA, Martin A, Singh S, Lillard J, Stiles J, Black CM, Igietseme JU. Molecular basis for the potency of IL-10-deficient dendritic cells as a highly efficient APC system for activating Th1 response. J Immunol. 2005;174:4860–4869. doi: 10.4049/jimmunol.174.8.4860. [DOI] [PubMed] [Google Scholar]
- 9.Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98:1142–1149. doi: 10.1182/blood.v98.4.1142. [DOI] [PubMed] [Google Scholar]
- 10.Westerberg L, Wallin RP, Greicius G, Ljunggren HG, Severinson E. Efficient antigen presentation of soluble, but not particulate, antigen in the absence of Wiskott-Aldrich syndrome protein. Immunol. 2003;109:384–391. doi: 10.1046/j.1365-2567.2003.01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 12.Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VL, Amigorena S. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 13.Ashe M, Pabon-Pena L, Dees E, Price KL, Bader D. LEK1 is a potential inhibitor of pocket protein-mediated cellular processes. J Biol Chem. 2004;279:664–676. doi: 10.1074/jbc.M308810200. [DOI] [PubMed] [Google Scholar]
- 14.Soukoulis V, Reddy S, Pooley RD, Feng Y, Walsh CA, Bader DM. Cytoplasmic LEK1 is a regulator of microtubule function through its interaction with the LIS1 pathway. Proc Natl Acad Sci U S A. 2005;102:8549–8554. doi: 10.1073/pnas.0502303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodwin RL, Pabón-Peña LM, Foster GC, Bader DM. The cloning and analysis of LEK1 identifies variations in the LEK/centromere protein F/mitosin gene family. J Biol Chem. 1999;274:18597–18604. doi: 10.1074/jbc.274.26.18597. [DOI] [PubMed] [Google Scholar]
- 16.Diwu Z, Zhang YZ, Haugland R. Novel Site-Selective Fluorescent Probes for Lysosome and Acidic Organelle Staining and Long-Term Tracking. Cytometry. 1994;52 Abstract #426B. [Google Scholar]
- 17.Harding C. Intracellular organelles involved in antigen processing and the binding of peptides to class II MHC molecules. Semin Immunol. 1995;7:355–360. doi: 10.1006/smim.1995.0040. [DOI] [PubMed] [Google Scholar]
- 18.Schuurhuis DH, Fu N, Ossendorp F, Melief CJ. Ins and outs of dendritic cells. Int Arch Allergy Immunol. 2006;140:53–72. doi: 10.1159/000092002. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Shehabeldin A, d Cruz LAG, Butler J, Somani AK, McGavin M, Kozieradzki I, d Santos AO, Nagy A, Grinstein S, Penninger JM, Siminovitch KA. Antigen Receptor–induced Activation and Cytoskeletal Rearrangement Are Impaired in Wiskott-Aldrich Syndrome Protein–deficient Lymphocytes. J Exp Med. 1999;190:1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calle Y, Chou HC, Thrasher A, Jones G. Wiskott-Aldrich syndrome protein and the cytoskeletal dynamics of dendritic cells. J Pathol. 2004;204:460–469. doi: 10.1002/path.1651. [DOI] [PubMed] [Google Scholar]
- 21.He Q, Martinez-Sobrido L, Eko FO, Palese P, Garcia-Sastre A, Lyn D, Okenu D, Bandea C, Ananaba GA, Black CM, Igietseme JU. Live-attenuated influenza viruses as delivery vectors for Chlamydia vaccines. Immunology. 2007;122:28–37. doi: 10.1111/j.1365-2567.2007.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]