Abstract
Earth's most recent major extinction episode, the Quaternary Megafauna Extinction, claimed two-thirds of mammal genera and one-half of species that weighed >44 kg between ≈50,000 and 3,000 years ago. Estimates of megafauna biomass (including humans as a megafauna species) for before, during, and after the extinction episode suggest that growth of human biomass largely matched the loss of non-human megafauna biomass until ≈12,000 years ago. Then, total megafauna biomass crashed, because many non-human megafauna species suddenly disappeared, whereas human biomass continued to rise. After the crash, the global ecosystem gradually recovered into a new state where megafauna biomass was concentrated around one species, humans, instead of being distributed across many species. Precrash biomass levels were finally reached just before the Industrial Revolution began, then skyrocketed above the precrash baseline as humans augmented the energy available to the global ecosystem by mining fossil fuels. Implications include (i) an increase in human biomass (with attendant hunting and other impacts) intersected with climate change to cause the Quaternary Megafauna Extinction and an ecological threshold event, after which humans became dominant in the global ecosystem; (ii) with continued growth of human biomass and today's unprecedented global warming, only extraordinary and stepped-up conservation efforts will prevent a new round of extinctions in most body-size and taxonomic spectra; and (iii) a near-future biomass crash that will unfavorably impact humans and their domesticates and other species is unavoidable unless alternative energy sources are developed to replace dwindling supplies of fossil fuels.
Keywords: paleoecology, pleistocene, mammal, ecological threshold
The Quaternary Megafauna Extinction (QME) killed >178 species of the world's largest mammals, those weighing at least 44 kg (roughly the size of sheep to elephants). More than 101 genera perished. Beginning ≈50,000 years (kyr) B.P. and largely completed by 7 kyr B.P., it was Earth's latest great extinction event. The QME was the only major extinction that took place when humans were on the planet, and it also occurred at a time when human populations were rapidly expanding during a global warming episode. Thus, the QME takes on special significance in understanding the potential outcomes of a similar but scaled-up natural experiment that is underway today: the exponential growth of human populations at exactly the same time the Earth is warming at unprecedented rates.
Causes of the QME have been explored primarily through analyzing the chronology of extinction, geographic differences in extinction intensity, timing of human arrival vs. timing of climate change, and simulations that explore effects of humans hunting megafauna (1–11). Results of past studies indicate that human impacts such as hunting and habitat alteration contributed to the QME in many places, and that climate change exacerbated it. Potentially added to those megafauna stressors was the explosion of a comet over central North America, which may have helped to initiate the Younger Dryas (YD) climatic event, and which may have caused widespread wildfires, although those ideas are still being tested (12).
Whatever the cause of the QME, one thing is clear: there was a dramatic change in the way energy flowed through the global ecosystem. The energy that powers ecosystems is derived from solar radiation, which is converted to biomass. Before the extinction, the energy needed to build megafauna biomass was divided among many species. After the extinction, increasing amounts and proportions of energy began to flow toward a single megafauna species, humans.
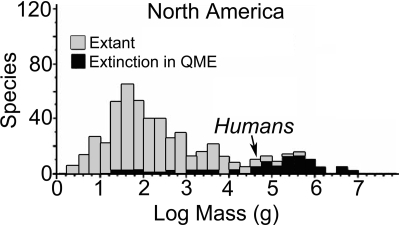
Humans are, by definition, a megafauna species, with an average body weight of ≈67 kg for modern Homo sapiens and 50 kg for stone-age people, placing us at the lower end of the body-size distribution for megafauna as a whole (Fig. 1). Previous work has demonstrated that, as human biomass grows, the amount of solar energy and net primary productivity (NPP) available for use by other species shrinks, ultimately shrinking the amount of the world's biomass accounted for by those non-human species (13–16). Therefore, growth of human biomass should be inversely related to biomass of other species in general and to other megafauna species in particular, given that large body size itself to a large extent depends on available NPP. Such energetically driven biomass tradeoffs provide a new way to explore the QME and have the potential of extracting general principles relevant to understanding the future. That is the approach I take here, one that necessarily has many caveats (see Methods), but that nevertheless leads to some interesting observations.
Fig. 1.
Body-size distribution of mammals in North America. The black bars illustrate the distribution of species that went extinct in the QME. Note that humans are at the lower end of the distribution for species that went extinct. Illustration modified from ref. 17; see that source for similar distributions of fauna from other continents.
Details of the QME and debates about its causes are summarized in recent reviews (2, 4, 10, 17). Salient points include the following. It was a time-transgressive extinction, beginning by 50 kyr B.P. in Australia and largely ending there by 32 kyr B.P., possibly concentrated in an interval between 50 and 40 kyr B.P. (9–11). In northern Eurasia and Beringia, extinctions were later and occurred in two pulses, the first between 48 and 23 kyr B.P. and the second mainly between 14 and 10 kyr B.P. (4), although some species lingered later in isolated regions (Irish elk until 7 kyr B.P. in central Siberia and mammoths until 3 kyr B.P. on Wrangel and St. Paul Island) (18, 19). In central North America, extinctions corresponded with the second Eurasia–Beringia pulse, starting at 15.6 kyr B.P. and concentrating between 13.5 and 11.5 kyr B.P. (4, 20). In South America, the extinction chronology is not well worked out, but growing evidence points to a slightly younger extinction episode, between 12 and 8 kyr B.P. (21).
Extinction intensity varied by continent, with Australia, South America, and North America hard-hit, losing 88% (14 extinct, 2 surviving), 83% (48 globally extinct, 2 extinct on the continent, 10 surviving), and 72% (28 globally extinct, 6 extinct on the continent, 13 surviving), respectively, of their megafauna mammal genera. Eurasia lost only 35% of its genera (4 globally extinct, 5 extinct on the continent, 17 surviving). Africa was little affected, with only 21% loss (7 globally extinct, 3 extinct on the continent, 38 surviving), including at least three Holocene extinctions.
Humans evolved in Africa, and hominins have been interacting there with megafauna longer than anywhere else. Insofar as they are dated, there is no correlation between human arrival or climate change for the few African extinctions. In general, extinctions in Australia intensified within a few thousand years of human arrival ≈50 kyr B.P. but did not correspond with unusual climate change. Extinctions in northern Eurasia corresponded in time with the first arrival and population expansions of H. sapiens, but both pulses also were concentrated in a time of dramatic climate change, the first pulse at the cooling into the Late Glacial Maximum (LGM) and the second pulse at the rapid fluctuation of YD cooling followed by Holocene warming (2, 4). Other species of Homo had been interacting with the megafauna for at least 400,000 years without significant extinctions before H. sapiens arrived. In Alaska and the Yukon, the first pulse of extinctions corresponded with LGM cooling but in the absence of significant human presence; the second pulse coincided with humans crossing the Bering Land Bridge and with the YD and Holocene climatic events. In central North America, extinction was sudden and fast, coinciding with the first entry of Clovis hunters, the YD–Holocene climatic transition, and the purported comet explosion. In South America, humans were already present by 14.6 kyr B.P., megafauna did not start going extinct until Holocene warming commenced some 11 kyr B.P., and species of ground-sloths, saber cats, glyptodonts, and horses have seemingly reliable radiocarbon dates as young as 8 kyr B.P. (21).
Few islands ever had non-human megafauna sensu stricto. That, and the fact that even human biomass of islands is very small compared with the continents, caused me not to consider them in this analysis. However, it is important to note that, on nearly every island where humans have landed, extinctions (especially of birds) and wholesale habitat destruction have shortly followed.
Results and Discussion
Species Loss vs. Human Population Growth.
The numbers of megafauna species lost were modest until the human growth curve began its rapid exponential rise between 15.5 and 11.5 kyr B.P. (Fig. 2). Then, species losses accelerated, primarily in the Americas, until the non-human megafauna baseline leveled off at 183 species, where it more or less remains today. However, human population continued to rise dramatically even after the counts of non-human megafauna species stabilized.
Fig. 2.
Number of non-human megafauna species that went extinct through time plotted against estimated population growth of humans.
Biomass Crash.
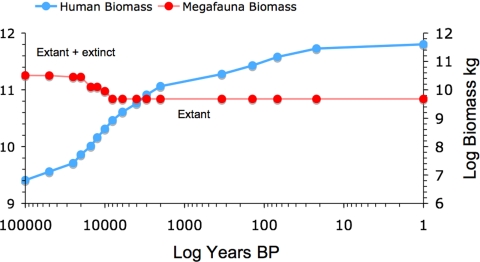
When converted to biomass, the inverse relationship between humans and non-human megafauna is evident (Fig. 3). Non-human megafauna biomass fell dramatically between 15.5 and 11.5 kyr B.P., concomitant with the initial steep rise in human biomass.
Fig. 3.
Estimated biomass of humans plotted against the estimated biomass of non-human megafauna. See Methods for parameters used.
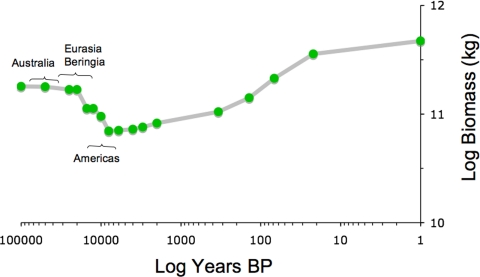
Summing the biomass calculated for humans and nonmegafauna species provides a way to track changes in overall megafauna biomass through time (Fig. 4). The results suggest that biomass loss from the early megafauna extinctions in Australia and the first pulse of extinctions in Eurasia and Beringia were almost exactly balanced by the gain in human biomass.
Fig. 4.
Change in the sum of human and non-human wild megafauna biomass through time. The brackets indicate when extinction pulses hit the respective geographic areas. See Methods for parameters used.
However, global megafauna biomass crashed dramatically between 15 and 11.5 kyr B.P. The crash reflects the second pulse of extinction in Eurasia–Beringia and the major extinction pulse in North and South America. This crash is evident in every one of the sensitivity tests, so it does not appear to be a computation artifact. The crash also remains evident when the biomass added by domestic species that support humans, pigs, sheep, goats, cattle, are included beginning 11 kyr B.P. Even using unreasonably high proportions of domesticates to humans (i.e., assuming today's proportions even at the dawn of animal domestication) fails to make the crash disappear.
Significantly, even though human biomass was rising dramatically at the time, that rise was not enough to balance the biomass lost from the megafauna that were going extinct. Therefore, more was at work than a simple biomass tradeoff among megafauna. The suddenness of the crash, its magnitude, and its distribution across three continents suggest a global threshold event (36, 37). Threshold events by definition are sudden changes to alternative ecosystem states induced either by some gradual change reaching a critical value or by abnormally strong perturbations. In either case, the net effect is to push the system from one “basin of attraction” (in this case, a world where megafauna body mass is distributed across many species) into a different one (a world where most megafauna body mass is concentrated around humans). The cause of the QME threshold event may well reflect a synergy of reaching a critical value of human biomass at the same time that ecologically unusual perturbations came into play. The unusual perturbations included increasingly sophisticated hunting of megafauna by people, habitat alteration by growing human populations, climate changes that would have decreased total global ecological energy at least temporarily, and possibly a comet impact.
The potentially dramatic effects of hunting (so-called overkill) have been most convincingly demonstrated by simulations of the effects of Clovis hunters first entering North America (1), which occurred just as global human biomass began to steeply rise. Those simulations suggest that hunting alone would result in many extinctions, because humans killed megafauna for food (1).
Similarly comprehensive simulations have not yet been done for other continents, but at least indirect human impacts (including habitat alteration and fragmentation) seem likely, given the coincidence of the megafauna biomass crash after first entry or markedly increasing population sizes of humans into various regions. These coincidences include first entry of humans into South America (near 14.6 kyr B.P.) and the entry and population growth of H. sapiens into Eurasia (from ≈40 kyr B.P.). Entry of humans also precedes the QME in Australia, although there, both were earlier than the worldwide biomass crash. In parts of Australia (38), North America (39), and South America (40), the evidence for indirect human impacts includes sedimentary records of increasing fire frequency, potentially indicating widespread habitat alteration through human-set fires. The indirect or direct role of humans in the QME also is suggested by the observation that the main megafauna survivors had habitat preferences that would have kept them farthest from humans (41).
Also coincident with the megafauna biomass crash was rapid climatic cooling, then warming as the YD gave way to the Holocene. In the Americas, where most of the extinction was concentrated, the tail end of the LGM, then the YD cooling, depressed NPP in at least the northern hemisphere. A slightly earlier YD-like cooling did the same in South America, just as humans began to interact with the non-human megafauna. Likewise, YD cooling was pronounced in northern Eurasia at the time of the world biomass crash.
If the evidence for a comet explosion over North America stands the test of time, NPP available to megafauna would have been further depressed at the time of the big extinction pulse. Large tracts of land are thought to have burned, and the explosion itself may have triggered the YD cooling in the northern hemisphere through opening the way for massive amounts of cold glacial meltwater to flood into the North Atlantic (12).
Biomass Recovery.
At the crash, megafauna biomass fell below its previous baseline value (Fig. 4). Then, beginning ≈10 kyr B.P., it began to build back up. By that time, the energy bottleneck that accompanied the crash was over. Global NPP was increasing as Holocene temperatures warmed, more land area was being exposed as glaciers melted, and there were fewer megafauna species on Earth among which to split the energy allocation. Even so, it took thousands of years for megafauna biomass to build back to precrash levels. The way it built back up was fundamentally different from the way it had been before, because virtually the entire recovery was by adding human biomass; the biomass of non-human megafauna remained virtually unchanged.
In terms of ecosystem dynamics under threshold models (36, 37), the biomass trajectory suggests that the global ecosystem crossed a threshold when the crash occurred. In the precrash state, megafauna biomass was distributed among many megafauna species, each with a relatively narrow ecological niche. In the postcrash alternative state, megafauna biomass concentrated in one species, humans, which has a very broad ecological niche. That means that ultimately humans were successful in coopting energy previously shared among other species with big bodies. It also means that not only are those extinct megafauna gone forever, but also there is no potential for new megafauna species to evolve into the “megafauna space” as long as humans are so abundant. In that respect, we have decreased biodiversity for as long as we remain abundant on Earth.
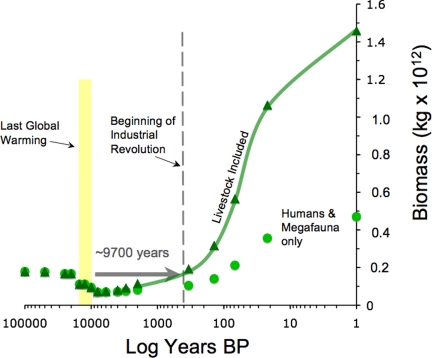
Recognizing the length of time it took the global ecosystem to recover to the precrash baseline depends on assumptions that were explored in the sensitivity tests. What I regard as the most reasonable input parameters result in the data illustrated in Fig. 5. That scenario includes domestic livestock, humans, and wild species as megafauna biomass and leads to two important observations.
Fig. 5.
Semilog plot of the sum of human and non-human wild megafauna (dots) and the sum of human, wild, and domestic megafauna (triangles connected by line). Yellow bar indicates the timing of the YD-Holocene climatic event that led into the current interglacial. See Methods for parameters used.
First, the buildup of human-associated megafauna biomass, even in the absence of the extinct megafauna, took ≈9,700 years to reach precrash levels. That indicates that recovering from global ecosystem shifts takes much longer than the shift itself. Even the sensitivity test that gives the fastest recovery time (unreasonably using large carnivore density equations for all species) requires 8,000 years to reach precrash megafauna biomass. The lesson is that if another threshold causes changes as dramatic as the QME, Earth's recovery will be far in the future, and not something the next few generations would see.
Second, the point at which biomass recovery is reached is very close to the beginning of the Industrial Revolution (Fig. 5) or at most 700 years before that (the sensitivity test noted above). This suggests that humans were unable to exceed the normal, precrash, solar-energy-limited baseline until we began to add to the global energy budget through mining fossil energy out of coal, oil, natural gas, and related sources. As soon as we began to augment the global energy budget, megafauna biomass skyrocketed, such that we are orders of magnitude above the normal baseline today.
Conclusions
When examined in the light of megafauna biomass tradeoffs, the cause of the QME becomes clearer, and implications for the future emerge. In essence, the QME begins to stand out not just as a major extinction event but also as an example of how threshold effects change the global ecosystem, and what new threshold events may be in sight.
In the specific case of the QME, a global crash in megafauna biomass resulted when the coincidence of at least two events constricted the share of ecological energy allotted to each non-human megafauna species. One event was a time of rapid growth in human biomass, which meant an inordinate supply of NPP began to be consumed by a single megafauna species. The other was a probable temporary reduction of NPP as the YD cooling hit both of the Americas and northern Eurasia (42). Exacerbating the global energetic constraints were the first entry of humans into the Americas, increasingly sophisticated hunting strategies and wider disruption of habitats, and possibly a comet explosion over North America.
In the general sense, the QME has four lessons. First, the global ecosystem is in a fundamentally different state than before the megafauna biomass crash. In contrast to the distribution of resources among the 350-plus megafauna species that were alive before the QME, most of the energy available to megafauna species in the post-QME world was coopted by humans. What is left after that is being subdivided among only 183 (plus or minus) other non-human megafauna species. It is perhaps comforting from a biodiversity standpoint that those other 183 species have remained on Earth since the crash. That may speak to a reasonable amount of stability in the alternative state the global ecosystem reached after the QME threshold event, at least in pre-Industrial times. It is also consistent with the expectations of ecological threshold theory.
Second, the Industrial Revolution elevated Earth's carrying capacity for megafauna biomass. However, despite that increase in carrying capacity, ≈50% (>90 species) of those megafauna species that persisted so well for the previous 10,000 years have become extinct, critically endangered, endangered, or vulnerable to extinction in the past few decades, including >40% of the megafauna species of mammals in Africa, the only continent that made it through the QME largely unscathed. For mammals as a whole, 25% of the 4,629 species known on Earth fall in the critically endangered through vulnerable categories. This suggests that not only has all of the “extra” carrying capacity been used by humans, but also we are beginning, as happened during the QME crash, to steal from the part of the global energy budget allotted to other megafauna species. We are also going farther and using energy previously allotted to species in even smaller body-size classes. Under business-as-usual scenarios, the inevitable result will be another biomass crash that moves down the body-size classes relative to the QME event.
Third, that the normal biomass baseline was exceeded only after the Industrial Revolution indicates the current abnormally high level of megafauna biomass is sustained solely by fossil fuels. If biodiversity is actually a tradeoff between human biomass and other species' biomass, as both the QME and theoretical considerations indicate (13–16), then depletion of fossil fuels without replacement by alternative energy sources would mean that a biomass crash is imminent, this one depleting human biomass and causing extinction in a wide spectrum of other species. Reliable projections on the number of years into the future that fossil fuels can sustain the global ecosystem at current levels vary, but generally are in the area of 50 more years for oil, 200 more years for natural gas, and 2,000 more years for coal (43). Thus, without technological breakthroughs, the next biomass crash could be in as little as a few human generations.
Fourth, it may be no coincidence that the QME did not occur until the intersection of growing human biomass and climate change that ultimately manifested as global warming. Climate change, either cooling or warming, itself produces adjustments in geographic range distribution and population size that can lead to extinction (44–49). Add to that the overall reduction of NPP that must have occurred with YD cooling, the indirect coopting of energy by rapidly growing human biomass, and direct human displacement of megafauna by killing and habitat alteration, and the combination becomes particularly lethal. Today, we stand at a similar crossroads, because growth of human biomass in the past few decades has moved us to the point where we are beginning to coopt resources from, further displace, and cause extinctions of species with whom we have been coexisting for 10,000 years. At the same time, Earth's climate is warming even faster than the rates of climate change that characterized the QME.
Recognizing the tradeoff between human biomass, non-human megafauna biomass, and non-human biomass in general highlights the need for extraordinary efforts to conserve the world's remaining biodiversity (16). Business as usual will not stave off severe biodiversity losses. The energetic constraints that underlie the biomass tradeoff mean that, as human biomass grows, the only way other species can persist is through conscious stepped-up efforts to save them, by such actions as setting aside reserves, enforced protection of existing reserves, and efficient and sustainable food-production practices. It is particularly urgent to act upon the knowledge that the high level of megafauna biomass today, which means humans, can be sustained only by developing alternative energy resources to replace the dwindling supply of fossil fuels.
Methods
Dating Conventions.
Unless otherwise noted, dates are expressed as calendar years before present (kyr B.P.).
Timing of Extinctions on Each Continent.
I used supporting information table 1 in ref. 4 to place the extinction of each megafauna species in one of the following temporal bins: <100 kyr B.P., 100–50 kyr B.P., 50–15.5 kyr B.P., 15.5–11.5 kyr B.P., and 11.5–0.5 kyr B.P. The latter bin is cut off at 500 years ago to exclude recent extinctions. As far as is known, before 500 years ago, the last megafauna species extinction was 3,000 years ago. Despite being somewhat coarse, these bins are adequate to examine the biomass tradeoff at the order-of-magnitude level of resolution to which the rest of the data are appropriate.
Estimating Human Biomass.
Hern (22) provided estimates of the numbers of hominins on Earth from the approximate first appearance of Homo habilis some 3 million years ago up to the number of H. sapiens projected to occur in approximately the year 2455. His estimates, based on calculating doubling times for hominin and human populations, were constrained by the fossil record of human evolution, by archaeological information, and by historical and demographic records up to 1999. I used his estimates for the numbers of people on Earth at a given point in time and multiplied that by the average weight of a person to estimate global human biomass for each time slice of interest. Following logic detailed in Hern (22), average weight for a human was considered 50 kg up to ≈400 years ago, and 67 kg thereafter.
Estimating Non-Human Megafauna Biomass.
In principle, megafauna biomass for a given species is calculated by multiplying the average body mass by the number of individual animals. To estimate this and produce the figures in this article, I used the following parameters. Average body mass values were taken from a recent compilation (23). For the few species not listed in that compilation, I used values for similarly sized animals that were listed. Number of individual animals per species was estimated in the following way. First, there is a correlation between body mass and population density, that is, individuals per km2 (24–26). To estimate density, I used regressions from ref. 25: for large herbivores, density = −0.44 × log(kg body mass) + 1.01; and for large carnivores, density = −1.31 × log(kg body mass) + 1.22. Second, megafauna species typically have geographic range sizes that average between 7% and 9% of the area of the continent on which they live. For Australia, I estimated the geographic range size of each megafauna species to be 7.8% of the continental area, or ≈600,000 km2 (27). For Africa, Eurasia, North America, and South America, geographic ranges sizes were set to 8.6%, 8.1%, 8.2%, and 7.2% of the respective continental areas (28–30). For each species, estimated density was multiplied by estimated geographic area to give an approximate number of individuals, which was then multiplied by estimated mass per individual.
Continental area was not constant through the time spanned by the QME, because during glacial times, nearly one-half of North America, about one-tenth of Europe and northern Asia, and a small percentage of South America were covered by glaciers. This loss of land was offset only to a very small extent by the exposure of currently submerged land with the lower sea level of glacial times. To account for varying continental area in the estimates of geographic range size, continental area during glacial times was considered to be 50% of its current size for North America, 90% of its current size for Europe and northern Asia, and 95% for South America. For the transitional time ≈10 kyr B.P., area for these continents was set at the intermediate values of 75%, 0.95%, and 98% of their current respective sizes.
Biomass of Domestic Stock.
To obtain a maximum value for the biomass of domesticated megafauna, I calculated the present proportion of human biomass to domestic stock biomass as tabulated by Hern (22). I then used that proportion to back-calculate the maximum biomass of domestic stock, given the estimated biomass of humans, going back to 10.5 kyr B.P., by which time pigs, goats, sheep, and cattle were first domesticated (31–35). For time slices up to 6 kyr B.P., only pigs, goats, and cattle were included in the domestic livestock count. More recent time slices also included horses, buffalo, camels, chickens, ducks, turkeys, and catfish. Clearly for prehistoric times, this method provides an overestimate of domestic stock biomass, because no one would argue that the first ranchers had as many domestic stock per person as is the case presently. However, because the purpose of this part of the analysis was to see whether domestic stock compensated for a reduction in wild megafauna, the overestimation actually makes the conclusions more robust.
Sensitivity Tests.
Sensitivity tests were conducted to assess how robust the general trends were to varying assumptions about density and geographic range size, on which the calculated biomass value for each species depends. Calculated density was varied by applying the regression equation for large carnivores (25) to the whole dataset at one extreme (results in least biomass), by applying the regression equation for large herbivores (25) to the whole dataset at the other extreme (results in most biomass), and by applying an average density equation to all species, density = −0.77 × log(g body mass) + 3.98 (24). One test also assumed a 10% increase in density of the megafauna that survived after the QME. Assumed geographic range size for each species was variably set between ≈9% and 5% of the area of the continent on which the species lived. Varying these parameters does not alter the general trend of biomass change through the QME. Varying them does affect the absolute values calculated for biomass and the amount of time indicated for biomass recovery but not in ways that obviate the main conclusions of this article.
Caveats.
Methods used here are intended to give simply an order-of-magnitude indication of how biomass changed through time and identify times of major biomass crash and recovery. The calculations are necessarily coarse. Exact values change given different inputs to the estimations, but the sensitivity tests make it seem unlikely that the important trends are simply estimation artifacts. Additional refinements would be desirable but are beyond the scope of this initial work. Such refinements ideally would include body mass vs. density regressions tailored to each species, refining the geographic range estimates for each species through niche modeling, and assessing details of the relationship among megafauna biomass, potentially available NPP, and available solar energy as estimated from climate and vegetation models. It would also be useful to accumulate region-by-region estimates of both human and non-human biomass through time. Despite leaving room for such refinements, this first effort highlights some overall trends that appear robust.
Acknowledgments.
I thank Elizabeth A. Hadly and Pablo Marquet for discussions about these ideas; Claudio Latorre for discussions about extinctions in South America; and Paul Koch, Robert Feranec, and Alan Shabel for help in assembling information. Useful comments on the manuscript were supplied by John Avise, Jenny McGuire, Nick Pyenson, and two anonymous reviewers. This work was done under the auspices of the U.S. Fulbright Senior Specialist Program at the Department of Ecology, Pontificia Universidad Católica, Santiago, Chile. Funding also was contributed by National Science Foundation Grants EAR-0720387 and DEB-0543641.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution II: Biodiversity and Extinction,” held December 6–8, 2007, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_biodiversity.
The author declares no conflict of interest.
References
- 1.Alroy J. A multispecies overkill simulation of the end-Pleistocene megafaunal mass extinction. Science. 2001;292:1893–1896. doi: 10.1126/science.1059342. [DOI] [PubMed] [Google Scholar]
- 2.Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. Assessing the causes of Late Pleistocene extinctions on the continents. Science. 2004;306:70–75. doi: 10.1126/science.1101476. [DOI] [PubMed] [Google Scholar]
- 3.Grayson DK, Meltzer DJ. A requiem for North American overkill. J Archaeol Sci. 2003;30:585–593. [Google Scholar]
- 4.Koch PL, Barnosky AD. Late Quaternary extinctions: State of the debate. Annu Rev Ecol Evol Syst. 2006;37:215–250. [Google Scholar]
- 5.MacPhee RDE, editor. Extinctions in Near Time: Causes, Contexts, and Consequences. New York: Kluwer Academic/Plenum; 1999. [Google Scholar]
- 6.Martin PS. Pleistocene overkill. Nat Hist. 1967;76:32–38. [Google Scholar]
- 7.Martin PS, Klein RG, editors. Quaternary Extinctions: A Prehistoric Revolution. Tucson: University of Arizona Press; 1984. [Google Scholar]
- 8.Martin PS, Wright HE Jr, editors. Pleistocene Extinctions: The Search for a Cause; Proceedings of the VII Congress of the International Association for Quaternary Research; New Haven, CT: Yale Univ Press; 1967. [Google Scholar]
- 9.Trueman CNG, Field JH, Dortch J, Charles B, Wroe S. Prolonged coexistence of humans and megafauna in Pleistocene Australia. Proc Natl Acad Sci USA. 2005;102:8381–8385. doi: 10.1073/pnas.0408975102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wroe S, Field J. A review of the evidence for a human role in the extinction of Australian megafauna and an alternative interpretation. Q Sci Rev. 2006;25:2692–2703. [Google Scholar]
- 11.Roberts RG, et al. New ages for the last Australian megafauna: Continent-wide extinction ≈46,000 years ago. Science. 2001;292:1888–1892. doi: 10.1126/science.1060264. [DOI] [PubMed] [Google Scholar]
- 12.Firestone RB, et al. Evidence for an extraterrestrial impact 12,900 years ago that contributed to the megafaunal extinctions and the Younger Dryas cooling. Proc Natl Acad Sci USA. 2007;104:16016–16021. doi: 10.1073/pnas.0706977104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer BA. Relating human population growth to the loss of biodiversity. Biodivers Lett. 1996;3:1–5. [Google Scholar]
- 14.Vitousek PM, Ehrlich PR, Ehrlich AH, Matson PA. Human appropriation of the products of photosynthesis. Bioscience. 1986;36:368–373. [Google Scholar]
- 15.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of earth's ecosystems. Science. 1997;277:494–499. [Google Scholar]
- 16.McDaniel CN, Borton DN. Increased human energy use causes biological diversity loss and undermines prospects for sustainability. Bioscience. 2002;52:929–936. [Google Scholar]
- 17.Lyons SK, Smith FA, Brown JH. Of mice, mastodons, and men: Human mediated extinctions on four continents. Evol Ecol Res. 2004;6:339–358. [Google Scholar]
- 18.Guthrie RD. Radiocarbon evidence of mid-Holocene mammoths stranded on an Alaskan Bering Sea island. Nature. 2004;429:746–749. doi: 10.1038/nature02612. [DOI] [PubMed] [Google Scholar]
- 19.Stuart AJ, Kosintsev PA, Higham TFG, Lister AM. Pleistocene to Holocene extinction dynamics in giant deer and woolly mammoth. Nature. 2004;431:684–690. doi: 10.1038/nature02890. [DOI] [PubMed] [Google Scholar]
- 20.Waters MR, Stafford TW. Redefining the age of clovis: Implications for the peopling of the Americas. Science. 2007;315:1122–1126. doi: 10.1126/science.1137166. [DOI] [PubMed] [Google Scholar]
- 21.Hubbe A, Hubbe M, Neves W. Early Holocene survival of megafauna in South America. J Biogeogr. 2007;34:1642–1646. [Google Scholar]
- 22.Hern WM. How many times has the human population doubled? Comparisons with cancer. Popul Environ. 1999;21:59–80. [Google Scholar]
- 23.Smith FA, et al. Body mass of late Quaternary mammals. Ecol/Ecol Arch. 2003;84:3401–3417. [Google Scholar]
- 24.Damuth J. Cope's rule, the island rule and scaling of mammalian populaton density. Nature. 1993;365:748–750. doi: 10.1038/365748a0. [DOI] [PubMed] [Google Scholar]
- 25.Silva M, Downing JA. The allometric scaling of density and body mass: A nonlinear relationship for terrestrial mammals. Am Nat. 1995;145:704–727. [Google Scholar]
- 26.White EP, Ernest SKM, Kerkhoff AJ, Enquist BJ. Relationships between body size and abundance in ecology. Trends Ecol Evol. 2007;22:323–330. doi: 10.1016/j.tree.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Murray BR, Dickman CR. Relationships between body size and geographical range size among Australian mammals: Has human impact distorted macroecological patterns? Ecography. 2000;23:92–100. [Google Scholar]
- 28.Letcher AJ, Harvey PH. Variation in geographical range size among mammals of the Palearctic. Am Nat. 1994;144:30–42. [Google Scholar]
- 29.Smith FDM, May RM, Harvey PH. Geographical ranges of Australian mammals. J Anim Ecol. 1994;63:441–450. [Google Scholar]
- 30.Brown JH. Macroecology. Chicago: University of Chicago Press; 1995. [Google Scholar]
- 31.Beja-Pereira A, et al. The origin of European cattle: Evidence from modern and ancient DNA. Proc Natl Acad Sci USA. 2006;103:8113–8118. doi: 10.1073/pnas.0509210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S-Y, et al. Origin, genetic diversity, and population structure of Chinese domestic sheep. Gene. 2006;376:216–223. doi: 10.1016/j.gene.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez H, Hughes S, Vigne J-D, Helmer D. Divergent mtDNA lineages of goats in an Early Neolithic site, far from the initial domestication areas. Proc Natl Acad Sci USA. 2006;103:15375–15379. doi: 10.1073/pnas.0602753103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larson G, et al. Ancient DNA, pig domestication, and the spread of the Neolithic into Europe. Proc Natl Acad Sci USA. 2007;104:15276–15281. doi: 10.1073/pnas.0703411104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedrosa S, et al. Evidence of three maternal lineages in near eastern sheep supporting multiple domestication events. Proc R Soc London Ser B. 2005;272:2211–2217. doi: 10.1098/rspb.2005.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheffer M, Carpenter SR. Catastrophic regime shifts in ecosystems: Linking theory to observation. Trends Ecol Evol. 2003;18:648–656. [Google Scholar]
- 37.Scheffer M, Carpenter SR, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;113:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 38.Miller GH, et al. Ecosystem collapse in Pleistocene Australia and a human role in megafaunal extinction. Science. 2005;309:287–290. doi: 10.1126/science.1111288. [DOI] [PubMed] [Google Scholar]
- 39.Burney GS, Burney LP, Burney DA. Landscape paleoecology and megafaunal extinction in southeastern New York state. Ecol Monogr. 2005;75:295–315. [Google Scholar]
- 40.Moreno PI. Climate, fire, and vegetation between about 13,000 and 9200 14C yr BP in the Chilean Lake District. Q Res. 2000;54:81–89. [Google Scholar]
- 41.Johnson CN. Determinants of loss of mammal species during the Late Quaternary ‘megafauna’ extinctions: Life history and ecology, but not body size. Proc R Soc London Ser B. 2002;269:2221–2227. doi: 10.1098/rspb.2002.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajdas I, Bonani G, Moreno PI, Arizteguic D. Precise radiocarbon dating of Late-Glacial cooling in mid-latitude South America. Q Res. 2003;59:70–78. [Google Scholar]
- 43.Galoppini E. Artificial Photosynthesis/Alternative Energy Sources. Eighteenth Annual US Kavli Frontiers of Science Symposium National Academy of Sciences; November 2–4, 2006; Irvine, CA. 2006. [Google Scholar]
- 44.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669. [Google Scholar]
- 45.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 46.Pounds JA, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 47.Root TL, Price JT, Hall KR, Rosenzweig C, Pounds JA. Fingerprints of global wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 48.Thomas CD, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 49.Barnosky AD. “Big game” extinction caused by Late Pleistocene climatic change: Irish elk (Megaloceros giganteus) in Ireland. Q Res. 1986;25:128–135. [Google Scholar]