Table 3. Combined summary data for additional clinical trials with exenatide.
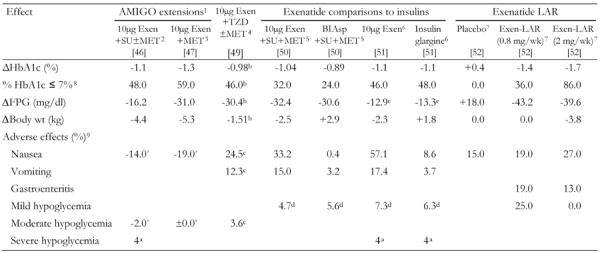
BID administration of exenetide in all studies with exenatide. Exen: exenatide. SU: sulfonylurea. MET: metformin. LAR: long-acting release. FPG: fasting plasma glucose. BIAsp: biphasic insulin aspart. BID: twice daily. 1 82 weeks; only included patients treated with exenatide [46, 47]. 2 Overweight patients in all groups. 39% of patients who entered the extended study withdrew. No antibody results reported. Nausea reported from weeks 30-40 to weeks 70-82 [46]. 3 Overweight patients. 43% of patients who entered the extended study withdrew. No antibody results reported. Adverse effects reported from weeks 30-40 to weeks 70-82 [47]. 4 16% of exenatide patients withdrew because of adverse events. Of the 115 (from 121 total) who were assessed, 40% were positive for anti-exenatide antibodies [49]. 5 21.3% of patients withdrew from the exenatide arm vs. 10.1% from the insulin BIAsp 70/30 arm. 45% were positive for anti-exenatide antibodies [50]. 6 19.4% of patients withdrew from the exenatide arm vs. 9.7% from the insulin glargine arm. 43% were positive for anti-exenatide antibodies [51]. 7 67% were positive for anti-exenatide antibodies [52]. 8 Fraction of patients (in %) with HbA1c ≤ 7% at the end of the study. 9 Fraction of patients with adverse effects (in %). In some cases, where marked with an asterisk (*), change of fraction of patients with adverse effects (in %) within the extended study (i.e. after completion of the first 30 wk study). a Cases (not percent). b Placebo-subtracted change. c Change treated patients vs. placebo. d Events per patient-year. e FPG reported as a 4 hour AUC in units of mmol/hr/l.
