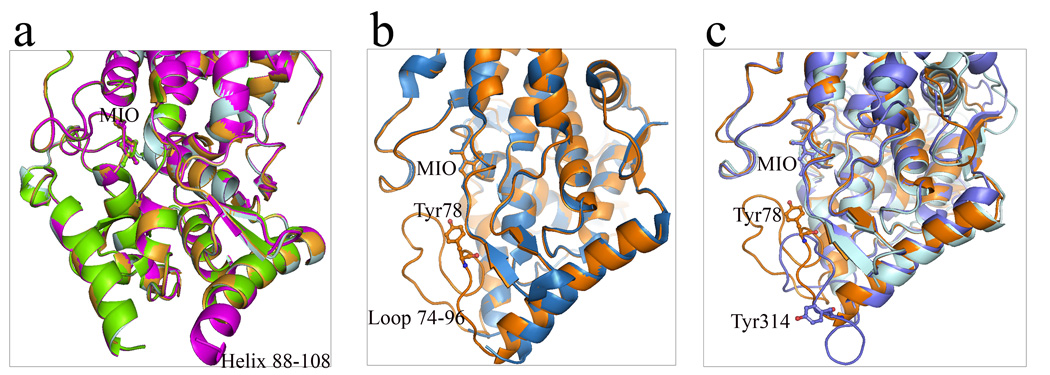
Figure 8.
Helix-loop conformational switch of aromatic ammonia lyases. (a) Superimposed monomer A, B, C and D of R. toruloides PAL (1Y2M) shows helix-loop switch in helix 88–108 region in monomer B. Monomer B: magenta; monomer A: pale cyan; monomer C: bright orange; monomer D: chartreuse. (b) Superimposed monomers of wild-type A. variabilis PAL (sky blue) and its double mutant (orange) presents disordered and well-ordered conformations of residues 75–91 region. (c) Three conformational forms of the active site loop 74–91 in A. variabilis PAL (loop-in position, orange) and its corresponding region in P. crispum PAL (loop-out position, slate) and R. toruloides PAL (disordered, cyan). In all figures, MIO, Tyr78 in A. variabilis PAL and Tyr110 in P. crispum PAL are shown in sticks.