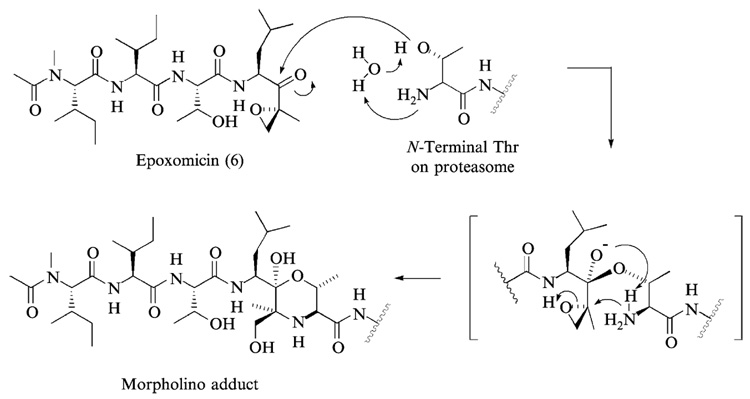
FIG. 3.
The mechanism of proteasome inhibition by epoxomicin is proposed on the basis of the x-ray structure of yeast 20S proteasome–epoxomicin complex. It is postulated that the unique specificity of epoxomicin is due to the formation of an unusual six-membered morpholino ring between Thr-1 of the catalytic subunit of 20S proteasome and the α′,β′-epoxyketone pharmacophore of epoxomicin.