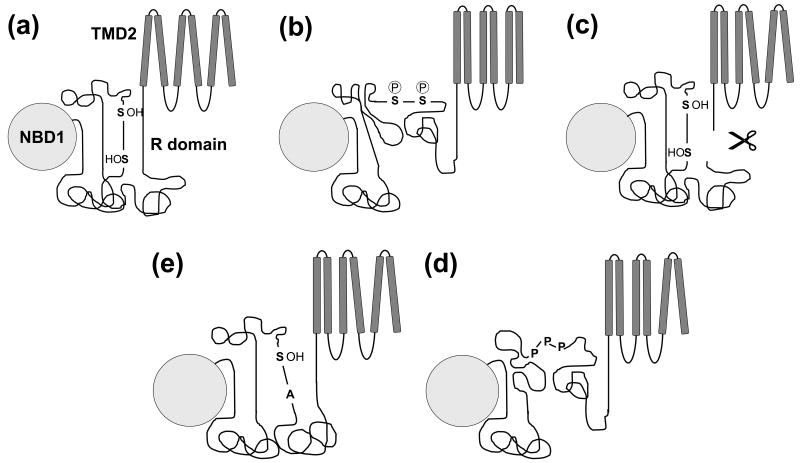
Figure 4. Working model for R domain function.
(a) In the non-phosphorylated state, R domain regions with higher ordered tendency form intramolecular interactions with the R domain itself and NBD1, maintaining a short distance between the two ends of the R domain inhibiting channel gating. Serines and threonines are important in the stabilization of this state. (b) Upon phosphorylation there is a net gain in negative charge and conformational changes within the R domain. All of these alterations cause reorientation of helices and signal transduction from the transmembrane to the nucleotide binding domains to promote gating. (c) Severing CFTR C-terminal of the R domain abolishes the distance constraint imposed by the R domain and leads to increased activation coupled to reorganization of helix packing. (d) The slightly increased activity of unphosphorylated Ser→Ala CFTR mutants may be caused by losing the stabilization provided by the hydroxyl amino acid, Serine. The alanine substitutions result in a more extended conformation of the R domain and therefore chloride channel activation. (e) The three prolines responsible for CFTR activation by PPIase are located in a region with higher ordered propensity. The basis of this alternative R domain activation pathway might be the weakened interaction between that region and other parts of the R domain or NBD1 after isomerization.