Abstract
The linker for activation of T cells (LAT) and the linker for activation of B cells (LAB/NTAL/LAT2) are integral proteins in receptor coupling to downstream events. Both proteins are expressed in natural killer (NK) cells and LAT is phosphorylated during target cell interactions or ligation of the immunoreceptor tyrosine-based activation motif (ITAM)–coupled CD16. Regardless, Lat−/− mice exhibit normal natural and antibody-mediated killing. Here we place both LAT and LAB in the DAP12 pathway of NK cells. Moreover, we unveil a LAT-independent pathway that requires expression of Syk. Mice lacking either LAT or LAB have a skewed Ly49 repertoire, and activated NK cells from Lat−/− mice have reduced responses to the ITAM-coupled receptor NK1.1. In contrast, resting Lat−/− NK cells show intact NK1.1 responses, whereas NK cells without LAB are hyperactive. Elimination of both adaptors severely reduces NK1.1 signaling under both conditions. Together these data show that NK ITAMs preferentially use a signaling cassette regulated by interplay between LAT and LAB. Activation by interleukin-2 causes a shift to greater dependency on LAT due to suppression of Syk signaling. The overlapping use of multiple adaptors permits fine-tuning of NK-cell ITAM responses over the course of an immune response.
Introduction
Natural killer (NK) cells play a central role in various immune responses including immune surveillance against tumor and/or virally infected cells. NK cells can directly lyse target cells and regulate immunity via the production of cytokines, most notably interferon-γ (IFNγ). Opsonized targets are recognized and lysed by NK cells via the low-affinity receptor for IgG (FcγRIIIA, CD16). In addition to CD16, NK cells use a variety of receptors that also use immunoreceptor tyrosine-based activation motifs (ITAM) including human NKp30, NKp44, and NKp46 and murine NK1.1 and NKp46.1 NK cell activation by these receptor complexes is regulated by the coexpression of a vast array of inhibitory receptors that facilitate the determination of “self” versus “nonself.” The best characterized of these are the human killer cell immunoglobulin-like receptors (KIR) and the murine Ly49 family of receptors.2 Originally described as inhibitory receptors, KIRs and Ly49s bind specific major histocompatibility complex (MHC) Class I molecules expressed on healthy cells and prevent lysis by attenuating signal transduction within the NK cell. The result is efficient lysis only of targets deficient in class I expression or that express high enough levels of activating ligands to override the inhibitory signal.
Interestingly, not all KIRs or Ly49s are inhibitory. Several members of each family lack characteristic inhibitory motifs and instead carry charged amino acids in their transmembrane domains that mediate interactions with the ITAM-containing signaling chain, DAP12.3 Engagement of ITAM-containing receptors in NK cells results in tyrosine phosphorylation of the ITAM, followed by recruitment and activation of protein tyrosine kinases of the Syk/ZAP70 family. This results in increased intracellular ionic calcium, activation of Erk-1 and -2 and ultimately IFNγ production and/or cytotoxicity.3–5
Studies of signal transduction in T cells have identified a variety of complex adaptor proteins, such as the linker for activation of T cells (LAT), that serve to link proximal tyrosine phosphorylation to downstream events.6–9 LAT is expressed in T cells, mast cells, and platelets and is rapidly tyrosine-phosphorylated in response to crosslinking of the T-cell antigen receptor (TcR), the high-affinity receptor for IgE on mast cells or GPVI on platelets.7,10,11 Upon receptor ligation, LAT associates with a variety of Src homology (SH) 2 domain containing proteins including phospholipase C (PLC)–γ1, Vav, SLP76, Grb2 and the 85 kDa regulatory subunit of PI3 kinase.7 Consistent with its proposed role as a linker between kinases and downstream effector proteins, cotransfection studies have identified LAT as a substrate of both Syk and ZAP-70 and T cells deficient in LAT are deficient in TcR-mediated Ras activation and calcium mobilization.8,12
LAT is expressed in NK cells and is phosphorylated upon crosslinking of CD16 or during interaction with susceptible, but not resistant, target cells.13,14 However, NK cells from Lat−/− mice maintain the ability to lyse typical NK susceptible target cells and their Fc receptor (FcR)–mediated ADCC is normal, showing that NK cells may have alternative means of coupling proximal phosphorylation to downstream outcomes.12 A likely candidate for an alternative activation pathway is the LAT-related molecule, linker for activation of B cells (LAB, also known as non–T-cell activation linker [NTAL]).6,9 Functional assays suggest that both LAT and LAB can be utilized by Ly49D15; however, no evidence directly ties these adaptors to ITAM signaling in NK cells.
We examined the possibility that NK cells might utilize both LAT and LAB as linkers between ITAMs and downstream events. Accordingly, we demonstrate that in both human and murine NK cells, engagement of DAP12-coupled receptors leads to tyrosine phosphorylation of LAT. Furthermore, we demonstrate that LAT is only essential for DAP12 signaling when Syk expression is low. This suggests ZAP70-mediated signals require LAT whereas Syk-mediated signals use an alternative pathway. In fact, we find that NK cells express high levels of LAB within their lipid rafts and that DAP12 signals induce its phosphorylation. ITAM signals from NK1.1 are enhanced in Lab−/− NK cells whereas Lat/Lab double-null NK cells are largely unresponsive implying negative regulation of NK cells by LAB. Therefore, NK cells use 2 parallel but crosstalking pathways consisting of ITAM-Syk-LAB/LAT and ITAM-ZAP70-LAT. NK-cell activation with interleukin-2 (IL-2) changes the dependency on these pathways. The result is NK cells resistant to disruption, poised to initiate the events critical in protection of the host from viral infection and/or stress-related injury.
Methods
Cell lines and NK-cell purification
The cell lines 721.221 and 721.221-Cw4 have been described.16,17 The RNKD2.38 line has been described4 and was maintained in RPMI-1640 containing 10% fetal calf serum, 2 mM l-glutamine, sodium pyruvate (1 mM), nonessential amino acids, 5 × 10−5 M 2-β mercaptoethanol and antibiotics (complete media). RNKDLS was derived from long-term culture of the RNKD2.38 parent line. High Ly49D expressing cells were sorted, and maintained in parallel with the parent line.
Human NK cells were isolated and expanded in culture as described.18 The purity of the NK cells was determined by flow cytometry using anti-CD56 and anti-CD3 (Beckman Coulter, Fullerton, CA) to be greater than 99% CD56+CD3− lymphocytes. The cells were heterogeneous for expression of CD16 and KIR epitopes and varied from donor to donor (data not shown). Experiments were performed using cells from days 8 through 21 of culture. Normal human lymphocytes were collected by the National Institutes of Health blood bank under blanket institutional review board approval.
Murine adherent lymphokine activated killer cells (ALAK) were purified as described.19 In some experiments, primary murine NK cells were isolated from splenocytes by positive selection using DX5 beads as per the manufacturer's directions (Miltenyi Biotec, Auburn, CA). Purified NK cells were expanded in vitro using compete media with 1000 U/mL IL-2.
Human NK cells were activated with HP-3E4 (IgM) ascites produced from the hybridoma, or purified antibodies IgM MOC104E (KIR2DL1, KIR2DS1, KI2DS1; Sigma-Aldrich, St Louis, MO), and FES172 (KIR2DS4; Beckman Coulter), or F(ab′)2 anti-CD16 (3G8; Medarex, Princeton, NJ). Monoclonal anti-Ly49D (4E5), anti-Ly49 C/I (5E6), anti-Ly49G2 (4D11), and anti-Ly49D/A (12A8) antibodies were purified from ascites and labeled as described.20 Anti-Ly49A (A1), anti-NK1.1 (PK136), and anti-CD3 (145-2C11) were purchased from BD Biosciences Pharmingen (San Diego, CA). F(ab′)2 anti-Ly49D/A (12A8) and F(ab′)2 anti-Ly49G2 (4D11) were prepared using pepsin digestion, purified with protein G and verified with SDS-PAGE. F(ab′)2 goat anti–rat IgG (KPL, Gaithersburg, MD) and Fab′2 goat anti-mouse IgG (Jackson Immunoresearch Laboratories, West Grove, PA) were used as crosslinkers. The anti-LAT and anti-LAB antibodies have been described.7,9,12 Anti–phospho-LAT/LAB antibody was from Millipore (Billerica, MA). Anti-PLCγ, anti-ZAP70, anti-Actin, and GST-Grb2 were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Syk (Fusion Antibodies, Belfast, United Kingdom) and anti-phosphotyrosine (4G10, Millipore) were used as described.4
Cell stimulation and immunoprecipitation.
For human NK-cell activation, 5 × 106 NK cells were incubated with primary antibody on ice for 5 minutes, washed once, secondary antibody was added, and the cells were incubated at 37°C for 2 minutes. The stimulation reaction was stopped by addition of ice-cold radio immunoprecipitation assay (RIPA) buffer (0.5% deoxycholic acid, 1% Triton X-100, 150 mM NaCl, 20 mMTrispH 8, 5 mM EDTA, 1 μg/mL aprotinin, 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 μg/mL pepstatin A, 5 mM sodium fluoride, and 2 mM sodium vanadate). For stimulation of sorted KIR2DS4+ NK cells, expanded NK cells were stained and sorted for expression of KIR2DS4 (FES172). After sorting, cells were washed and resuspended in cold Dulbecco phosphate buffered saline (DPBS). Cells were incubated at 37°C for the indicated times in the presence of 5 μg goat anti–mouse crosslinking antibody. Controls were stimulated without secondary antibody or cells stimulated for 10 minutes with pervanadate. After stimulation, cells were lysed in lauryl-maltoside buffer (1% laurylmaltoside in 20 mM Tris [pH 7.5], 100 mM NaCl, 10% glycerol, 10 mM EDTA, 0.4 mM Na3VO4, aprotinin, leupeptin, and PMSF). Postnuclear lysastes were immunoprecipitated with 2 μL anti-LAT antisera or 4 μL anti-LAB antisera and collected with protein G–coupled agarose beads. Immunoprecipitates were separated by SDS-PAGE under nonreducing conditions, transferred to polyvinylidene fluoride (PVDF; Millipore) membrane, and analyzed by Western blot. To confirm loading, filters were stripped and reprobed with anti-LAT antibody.
RNKD2.38 and RNKDLS stimulation, lysis, and protein immunoprecipitation were performed as described.4 Lysates were clarified by centrifugation, then immunoprecipitated for 2 to 3 hours at 4°C with anti-LAT antibody prebound to Protein A Sepharose (Invitrogen, Carlsbad, CA) or 2 μg of GST or GST-Grb2 fusion protein (Santa Cruz Biotechnology) bound to glutathione sepharose beads (GE Healthcare, Little Chalfont, United Kingdom). Complexes were washed with RIPA and proteins were eluted in Laemmli buffer as noted and separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to Immobilon-P and then blotted with either anti-LAB or anti-phosphotyrosine as above. In some experiments whole cell lysates were resolved by SDS-PAGE and immunoblotted with anti-phospho-Erk1 and 2 or pan-Erk1 and 2 as described by the manufacturer (Cell Signaling Technology, Danvers, MA).
Vaccinina infections.
Recombinant vaccinia virus expressing Ly49D has been described.21 For vaccinia-derived expression of Ly49D in human NK cells, the cells were infected for 20 hours with 20 plaque-forming units (pfu)/cell semipurified vaccinia as previously described.22 The cells were washed once with Iscove media and a small aliquot removed for flow cytometry. The cells were resuspended in 100 μL of media and incubated on ice for 10 minutes, then activated as above.
WR′ vaccinia and recombinant vaccinia expressing Syk were the gift of Dr Andrew Scharenberg (University of Washington, Seattle). LAT-expressing vaccinia was generated by cloning a full-length LAT cDNA into the pSC66 vaccinia plasmid recombined into the thymidine kinase (TK) locus of WR′ as previously described.23,24 Virus was amplified in TK cells and released from the cells by sonication. The titers of plaque-forming units per milliliter (pfu/mL) were determined on TK cells. RNKDLS cells were infected at an MOI of 10 to 20 with WR′ or recombinant vaccinia virus for 4 hours in serum-free media. After washing, an aliquot of cells was removed for Western analysis and the remaining cells were loaded with Fluo3-AM and Fura-RedAM (Invitrogen), and calcium mobilization was determined as described.4
Mice.
Lat1−/− mice were the generous gift of Dr Connie Summers and Dr Lawrence Samelson (National Cancer Institute, Bethesda, MD) and have been described.12 Lat1−/− mice were backcrossed to C57BL/6 mice for 10 generations before use. Microsatellite marker analysis demonstrated more than 98% C57BL/6 chromosome content (data not shown). Lat2−/− (the gene encoding LAB/NTAL) mice were backcrossed to C57BL/6 mice for 8 generations at Duke University (Durham, NC).25 Mice null for both Lat1 and Lat2 were generated by intercrossing the Lat1−/− and Lat2−/− lines.12,25 For clarity we refer to Lat1 and Lat2 as LAT and LAB, respectively, throughout. Controls were C57BL/6 bred in parallel at the National Cancer Institute-Frederick. All experiments were conducted with the approval of the Animal Care and Use Committee of the National Cancer Institute-Frederick and were maintained in a dedicated pathogen-free environment. Animal care was provided in accordance with the procedures outlined in “A Guide for the Care and Use of Laboratory Animals.”26
Intracellular IFNγ analysis and ribonuclease protection assays.
ALAK or purified murine NK cells were plated onto wells precoated with 1 μg/mL PK136 or control IgG in complete medium supplemented with 1 ug/mL Brefeldin A. After 5 hours of culture at 37°C, cells were harvested and stained with PE-conjugated anti-DX5 and PerCPcy5.5-conjugated anti-CD3. The cells were washed and fixed and permeablized using BD CytoFix/Cytoperm solution. After washing, Fc receptor was blocked by incubation with 1 μg/sample anti-FcRγI/II (2.4G2) and cells were stained with APC-conjugated anti-IFNγ (Clone XMG1.2; BD Pharmingen). NK cells, as defined by DX5+CD3− lymphocytes, were gated and analyzed for intracellular IFNγ staining. Ribonuclease protection assays were performed using 33P-labeled mMCK1 and MCK5C multiprobes. Each assay was performed according to the manufacturer's specifications (Pharmingen) with modifications as previously described.27
Results
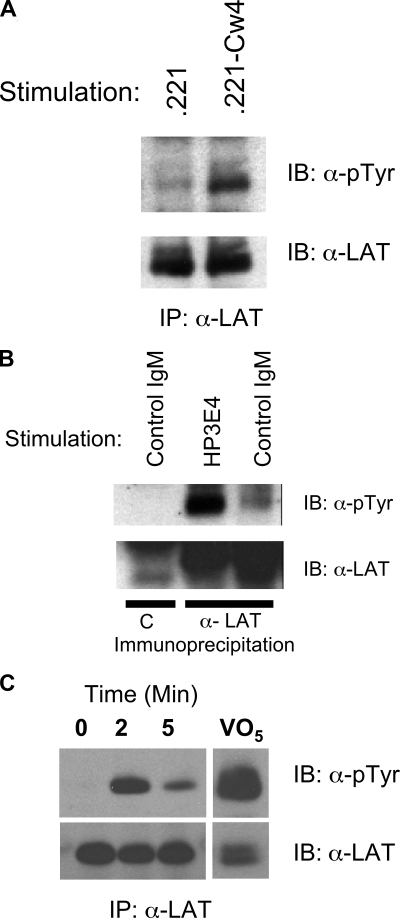
We and others have demonstrated calcium mobilization and cytokine induction after crosslinking of DAP12-coupled activating KIR and Ly-49s on NK cells. Therefore, we examined the involvement of LAT in transmitting DAP12-mediated signals of activating KIR. Human NK cells were mixed with either the class I–deficient target cell 721.221 or 721.221 transfected with HLA-Cw4, a ligand for KIR2DS1.28 In at least 3 independent donors, LAT phosphorylation was greater when the target cells expressed Cw4 (Figure 1A). These data suggest that DAP12-coupled receptors that bind class I MHC might facilitate LAT phosphorylation.
Figure 1.
Induction of LAT phosphorylation in human NK cells by CD16, target cells, or anti-KIR/KAR antibody. (A) Human NK cells (5 × 106) were incubated with Class I–deficient 721.221 (.221) targets or targets transfected with HLA-Cw4 (.221-Cw4) cells for 10 minutes at 37°C. Phosphorylation of immunoprecipitated LAT was determined by Western blot (pTyr, top panel) and the amount of LAT in each lane verified with anti-LAT (bottom panel). (B) Human NK cells (5 × 106) were stimulated for 10 minutes at 37°C with a control IgM (MOPC104E) or anti KIR/KAR (HP-3E4). The samples were analyzed as in A except the first sample was immunoprecipitated with preimmune serum = C. (C) IL-2 expanded primary human NK cells were sorted for expression of KIR2DS4. Immediately after sorting the cells were stimulated by warming to 37°C in the presence of anti–mouse crosslinking antibody for the indicated times. Control cells had crosslinking antibody added after lysis. Phospho-LAT was assayed as in panel A.
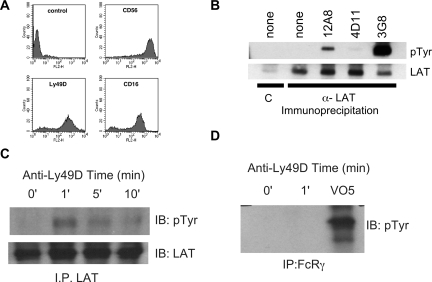
Next we directly stimulated activating KIR using IgM HP-3E4 (Figure 1B). Incubation of NK cells from several donors with HP-3E4 alone induced LAT tyrosine phosphorylation despite the ability of HP-3E4 to bind to both activating and inhibitory molecules on these cells. However, the mixed expression of these receptors on NK cells prevented the demonstration of LAT phosphorylation exclusively via DAP12-coupled receptor complexes. Therefore, we sorted KIR2DS4 expressing IL-2 expanded primary NK. Immediately after sorting, we crosslinked cell-bound anti-KIR2DS4 with goat anti–mouse antibody then immunoprecipitated LAT and immunoblotted with anti-phosphotyrosine. This approach confirmed rapid, transient phosphorylation of LAT downstream of DAP12 (Figure 1C). As a last test of the ability of human DAP12-coupled receptors to utilize LAT, we introduced the DAP12-coupled mouse NK cell receptor, Ly49D, into human NK cells using recombinant vaccinia virus. Vaccinia virus exposure resulted in Ly49D expression on 70%–80% of NK cells (Figure 2A). Ly49D was crosslinked using F(ab′)2 fragments of an antibody specific for Ly49D (12A8), or as a control, antibody to an inhibitory Ly49 (Ly49G, 4D11). Treatment of Ly49D-expressing NK cells with 12A8, but not the control F(ab′)2, resulted in robust tyrosine phosphorylation of LAT (Figure 2B), demonstrating that LAT is directly involved in DAP12-coupled signaling in human NK cells. Stimulation with anti-CD16 (3G8) served as a positive control.
Figure 2.
Induction of LAT phosphorylation by Ly49D expressed in human NK cells and RNKD2.38. Human NK cells were infected overnight with vaccinia carrying the Ly49D gene. (A) Vaccinia infected cells were stained for expression of CD56, Ly49D and CD16 and analyzed using flow cytometry. (B) The infected NK cells were divided into 5 samples and stimulated for 2 minutes at 37°C with the F(ab′)2 antibodies as indicated. Phosphorylation of immunoprecipitated LAT was determined by Western blot (pTyr, top panel) and the amount of LAT in each lane verified with anti-LAT (bottom panel). Controls were immunoprecipitated with preimmune sera = C. (C) RNKD2.38 cells (107) were stimulated with anti-Ly49D antibody (4E5), for 1, 5, or 10 minutes at 37°C. Phosphorylation and LAT expression were determined as in panel B. (D) FcϵRIγ was immunoprecipitated from RNKD2.38 cells stimulated with anti-Ly49D (4E5) or pervanadate (VO5). Proteins were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine. Results are representative of at least 2 independent experiments.
As a final demonstration of LAT involvement in the DAP12 signaling, we used a rat NK-cell line engineered to express Ly49D, RNKD2.38.4 We have previously demonstrated that Ly-49D physically and functionally interacts with endogenous rat DAP12 in RNKD2.38. When Ly49D was crosslinked on RNKD2.38 with either mAb 12A8 (data not shown) or mAb 4E5, rapid and transient LAT phosphorylation was readily detected (Figure 2C). In both cases LAT phosphorylation was near maximal at 1 minute and began to decline shortly thereafter. Importantly, this 4E5-directed LAT tyrosine phosphorylation was not due to coengagement of the rat Fc receptor because F(ab′)2 fragments of 4E5 also induced LAT phosphorylation (data not shown). Moreover, immunoprecipitation of FcγRI gamma chain after Ly49D crosslinking demonstrated no significant phosphorylation (Figure 2D). These data confirm a direct connection between DAP12-coupled signaling and LAT in NK cells. Taken together with the described involvement of LAT in FcR signaling in NK cells, our data demonstrate that multiple ITAM-based signals in NK cells use this adaptor.
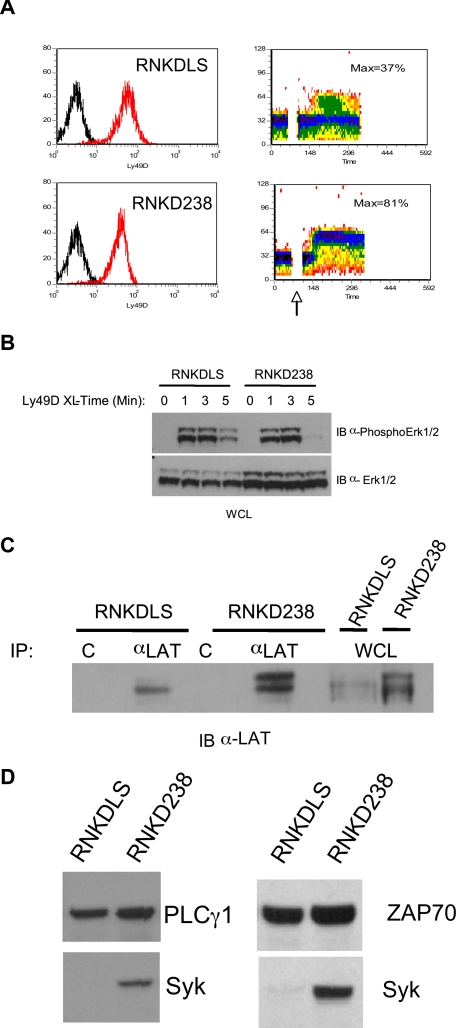
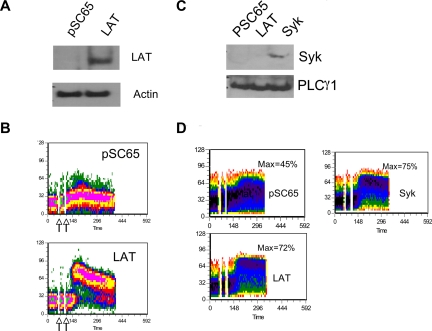
We further investigated the role of LAT in NK ITAM-mediated signaling through analysis of an RNKD2.38 derivative. This line, known as RNKDLS, is a spontaneously arising RNKD2.38 variant that expresses high levels of Ly49D but fails to mobilize calcium efficiently upon ligation of this receptor (Figure 3A). In contrast, Ly49D engagement on RNKDLS efficiently up-regulates mitogen-activated protein kinase (MAPK) activation (Figure 3B), suggesting a specific block in ITAM-mediated calcium mobilization. Given our evidence of LAT involvement in DAP12 signaling, we assayed the levels of various signaling proteins hypothesized to be involved in calcium mobilization downstream of ITAMs in NK cells. This analysis demonstrated a significant reduction in the levels of LAT (Figure 3C) and Syk (Figure 3D) in RNKDLS relative to RNKD2.38, despite normal levels of PLCγ1 and ZAP70 (Figure 3D). These data demonstrated that Syk and LAT were largely dispensable for ITAM-mediated MAPK activation but were involved in calcium mobilization. Reconstituting LAT expression completely restored calcium mobilization in RNKDLS confirming the ability of NK cells to bypass the need for Syk in ITAM signaling (Figure 4A,B). Unexpectedly, reconstitution of Syk in RNKDLS also restored calcium mobilization despite the low levels of LAT in these cells (Figure 4C,D). It is important to note that reexpression of Syk did not result in increases in LAT (not shown), nor did expression of LAT increase expression of Syk (Figure 4C). These data suggest that when Syk is expressed in NK cells, ITAM signaling is largely independent of LAT. When Syk is limiting, LAT is absolutely required to integrate a DAP12 signal.
Figure 3.
Characterization of RNKDLS. (A) RNK2.38 or RNKDLS were stained with isotype control (black line) or anti-Ly49D (red line) and analyzed for Ly49D expression by FACS (left panels). Similar aliquots of cells were analyzed for calcium mobilization after stimulation with anti-Ly49D (4E5) and crosslinking antibody. (B) RNKD2.38 and RNKDLS were stimulated with anti-Ly49D (4E5) for the time indicated. Whole cell lysates were then probed with anti-phospho-Erk1/2 (top panel) or total Erk1/2 (bottom panel). (C) Lysates of resting RNKD2.38 or RNKDLS were immunoprecipitated with control antibody [C] or with anti-LAT and immunoblotted with anti-LAT (lanes 1-4). Whole cell lysates (WCL) from RNKD2.38 and RNKDLS were immunoblotted with anti-LAT (lanes 5,6). (D) Equal amounts of whole cell lysates of RNKDLS and RNK2.38 were immunoblotted for the indicated signaling proteins. Results are representative of at least 2 independent experiments.
Figure 4.
Reexpression of LAT or Syk restores calcium mobilization in RNKDLS. RNKDLS cells were infected with control Vaccinia virus (pSC65) or recombinant virus expressing LAT or Syk, as indicated. (A) Aliquots of cells were removed for immunoblotting with LAT and actin, and (B) remaining cells were loaded with calcium-sensitive dyes and assayed for calcium mobilization after treatment with anti-Ly49D (4E5) and crosslinker. (C) In a second experiment, RNKDLS cells were infected as indicated. Similar to panel A, postnuclear cell lysates were immunoblotted for Syk or PLCγ1 as indicated and (D) remaining cells were assayed for calcium mobilization as in panel B. Max% indicates maximum percentage of cells responding.
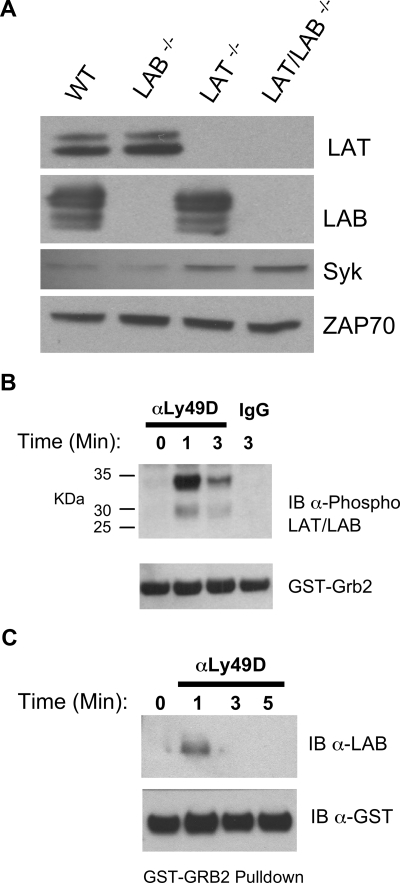
The ability of RNKDLS to signal when Syk levels were restored suggested the existence of a parallel, overlapping signaling cassette functional in the absence of LAT. Consistent with this notion, the expression of LAB in NK cells has been reported and suggested to be sufficient for IFNγ production by Ly49D/DAP12.15 Moreover, these authors demonstrated that culture in IL-2 reduced the levels of LAT mRNA in NK cells. Our analysis of LAT, LAB, Syk, and ZAP70 expression in T-depleted ALAK cells demonstrated readily detectable levels of both LAT and LAB and suggested no compensatory increases in LAT or LAB when the other is missing (Figure 5A). Interestingly, this analysis revealed increased Syk expression in ALAK lacking LAT. LAB was also detected in RNKD2.38 and both LAT and LAB were enriched within the cholesterol rich raft fractions of murine ALAK and RNKD2.38 (data not shown). Stimulation of RNKD2.38 with pervanadate followed by incubation with a Grb2 fusion protein revealed that phospho-LAB derived from NK cells interacts with multiple signaling proteins (data not shown). Therefore, we analyzed Grb2 interacting proteins from RNKD2.38 stimulated with anti-Ly49D. Immunoblotting with anti–phospho-LAT and phospho-LAB29 revealed that both these proteins interacted with Grb2 in a stimulation dependent manner. These data suggested that although both LAT and LAB are utilized in intact NK cells, LAT was the primary target of the receptor complex (Figure 5B). Consistent with this notion we could not detect phospho-LAB in the lysates of KIR-stimulated primary NK cells (data not shown). GST-Grb2 pulldowns of lysates from anti-Ly49D stimulated Lat−/− ALAK cells, however, directly demonstrated utilization of Lab in DAP12 signaling (Figure 5C).
Figure 5.
Expression and utilization of LAB in RNKD2.38 and LAT−/− NK cells. (A) Equal amounts of protein from lysates of T cell–depleted ALAK cells from the indicated strains were directly immunoblotted with anti-Syk or anti-ZAP70 as indicated (bottom 2 panels). The same lysates were also immunoprecipitated, then immunoblotted with anti-LAT or anti-LAB as indicated (top 2 panels). (B) RNKD2.38 were stimulated with anti-Ly49D (4E5) for the indicated time and lysates were incubated with GST-Grb2. Bound proteins were resolved by SDS-PAGE and immunoblotted with anti–phospho-LAT/LAB. (C) LAT−/− ALAK cells were stimulated and incubated with GST-Grb2 as in B, and immunoblotted with anti-LAB. Results shown are representative of 2 or more independent experiments.
To confirm the involvement of LAT and LAB in primary NK cells we studied receptor expression in Lat−/−, Lab−/−, and Lat/Lab double-null mice. As expected, both Lat−/− and Lat/Lab double-null mice had higher numbers of NK cells than wild-type or Lab−/− animals. Surprisingly, double-null mice had a lower percentage of NK cells in the spleen than Lat−/− mice, resulting in lower overall NK-cell numbers (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Although Chiesa et al detected no changes in the Ly49 repertoire,15 in multiple independent experiments we did. The data in Table 1 demonstrate that Ly49C/I was reduced in both Lat−/− and Lab−/− mice but the effect was not cumulative in double-null cells. Ly49A was reduced on Lab−/− cells even further reduced on Lat−/−, and almost absent on double-null NK cells. Ly49G2 expression was reduced on Lab−/− NK cells and slightly increased on Lat−/− cells, and these effects appeared to cancel one another out. Lastly, Ly49D was reduced in Lat−/− and double-null NK but unaffected in Lab−/− NK suggesting a specific LAT effect. Even though the percentage of NK cells expressing some Ly49s changed, the staining intensity (MFI) of Ly49s was not altered. In contrast to the Ly49s, NK cell expression of CD244 and NK1.1 was unaffected and analysis of NK cells for CD122, CD127, CD94, and NKG2ACE revealed only minor differences between strains (Table S1).
Table 1.
Altered expression of Ly49s in Lat−/−, Lab−/−, and Lab/Lat−/− NK cells
| Ly49 | WT | LAT−/− | LAB−/− | LAB/LAT−/− |
|---|---|---|---|---|
| Ly49D (4E5)* | 50.4 ± 1.9† | 25.9 ± 1.8* | 47.8 ± 3.3 | 25.3 ± 1.1‡ |
| Ly49G (4D11) | 44.2 ± 0.5 | 52.6 ± 1.2* | 35.0 ± 1.7* | 41.8 ± 1.0 |
| Ly49C/I (5E6) | 46.8 ± 2.5 | 28.0 ± 2.9* | 38.7 ± 3.4 | 23.6 ± 1.7* |
| Ly49A (YE148) | 11.7 ± 0.9 | 4.8 ± 0.3* | 6.6 ± 0.8* | 2.5 ± 0.4* |
| Ly49A (A1) | 14.4 ± 0.9 | 5.7 ± 0.6* | 9.7 ± 0.7* | 3.8 ± 0.2* |
WT indicates wild-type.
Antibody used for the indicated Ly49.
Data reflect the percentage of NK1.1+CD3− NK cells expressing the indicated Ly49 plus or minus SEM and reflect 3 to 5 independent analyses.
P < .01 relative to WT by Student 2-tailed t test.
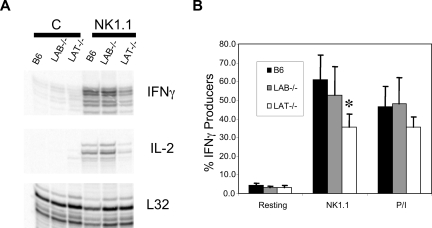
Despite the skewed expression of Ly49s in Lat−/− and Lab−/− ALAK, their expression of NK1.1 was identical and NK1.1 is functionally coupled to the ITAM-containing chain, FcϵRIγ. Therefore, we assessed the ability of NK1.1 to induce cytokine mRNA in NK cells lacking LAT or LAB.30 ALAK cells from wild type, Lab−/−, or Lat−/− mice were plated onto stimulating concentrations of anti-NK1.1. After 6 hours, the cells were harvested and assayed for cytokine and chemokine mRNA by RNAse protection assay. Despite normal surface expression of NK1.1 on all populations, after NK1.1 stimulation Lat−/− ALAK were less capable of up-regulating IFNγ, IL-2 (Figure 6A), or MIP1α and MIP1β (Figure S2) than either wild-type or Lab−/− ALAK cells. Importantly, although Lat−/− ALAK cells responded less than wild-type or Lab−/− cells, they did produce cytokine and chemokine mRNA, suggesting ITAM signals in the complete absence of LAT. These findings were confirmed by intracellular staining for IFNγ (Figure 6B). The observation that IFNγ production induced by PMA and Ionomycin was normal in Lab−/− ALAK but slightly lower in Lat−/− cells perhaps indicates a developmental defect in the latter cells. Lastly, preliminary analysis of ALAK cells generated from SCID mice confirmed that the defect in Lat−/− cytokine production was not due to the aberrant expansion of Lat−/− NK cells in vivo (Figure S3).
Figure 6.
LAT−/− ALAK cells have reduced levels of cytokine production. (A) ALAK cells from the indicated strains were stimulated with anti-NK1.1 or not “C” for 5 hours. mRNA was extracted and subjected to RPA analysis for IFNγ and IL-2 as described in “Methods.” (B) The indicated ALAK cells were stimulated for 6 hours with plate bound anti-NK1.1 or a combination of PMA and ionomycin in the presence of befeldin A. The cells were then stained for DX5, CD3, and intracellular IFNγ. The percentage of DX5+CD3− cells producing IFNγ is shown. Data in panel A are representative of 3 independent experiments. Data in panel B are the mean of 3 independent experiments plus or minus SEM. *P < .03 by Student t test between B6 and LAT−/−.
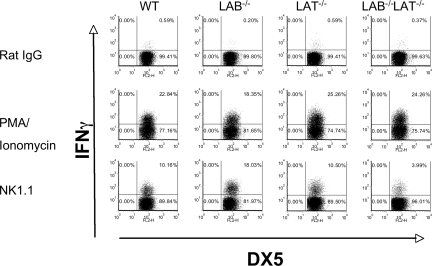
Our detection of LAB phosphorylation after ITAM stimulation of murine NK cells, together with the residual NK1.1-induced cytokine and chemokine mRNA, suggests significant compensation by LAB in Lat−/− NK cells. We tested this possibility by studying IFNγ production in Lat/LAB double-null mice. When we stimulated freshly isolated NK cells from wild-type, Lat−/− Lab−/−, or double-null mice with NK1.1 we found that NK cells lacking LAB were more likely to produce IFNγ than those of wild-type mice. In contrast to our findings with ALAK cells, freshly isolated Lat−/− NK cells did not show reduced IFNγ production. Lastly, these data showed that Lat/LAB double-null mice have significantly reduced, but not a total absence of IFNγ production (Figure 7). All the cell populations produced IFNγ in response to PMA and Ionomycin demonstrating their ability to produce the cytokine once proximal signaling events are bypassed. These data suggest that in the absence of IL-2 expansion, LAB can fully compensate for the lack of LAT and that similar to the findings in mast cells, LAB tempers LAT-mediated activation in resting NK cells.25
Figure 7.
LAB−/− resting NK cells are hyperresponsive to NK1.1 stimulation, whereas LAB/LAT−/− NK cells are hyporesponsive. (A) Freshly purified DX5+ cells were isolated from spleens of the indicated strains. The cells were stimulated with rat IgG, anti-NK1.1, or PMA and ionomycin and assayed for intracellular IFNγ as in Figure 6. The data presented are representative of 3 independent experiments.
Discussion
Several biochemical studies have examined the proximal events associated with the ligation of DAP12-coupled receptors in NK cells.3–5,31 This work has established the initial use of members of the Src-family of protein tyrosine kinases, followed by recruitment and activation of either Syk or ZAP70. Downstream biochemical events associated with ligation of activating KIR and/or Ly49s have also been described including the utilization of Vav proteins, activation of the MAP kinase cascade, and increases in intracellular calcium mobilization via the activation of phospholipase Cγ-1 and/or -2.3,4,32 Here, for the first time we describe the proteins and mechanism that facilitate the connection between these proximal and distal events in NK cells. Our findings demonstrate the cooperative and partially redundant use of the adaptors LAT and LAB in the signaling cascade of the ITAM containing receptor complexes Ly49D/DAP12 and NK1.1/FcRγ. Perhaps more importantly, our data provide evidence supporting the existence of 2 distinct signaling cassettes, one composed of ITAM:Syk:LAB/LAT and the other of ITAM:Zap70:LAT. Although the LAT cassette is dominant in a fully competent NK cell, both of these cassettes can operate, making the cells resilient to insult to one or the other pathway.
Dual utilization of LAT and LAB in NK ITAM signaling
Our demonstration that LAT is directly phosphorylated in response to DAP12 signaling in NK cells represents the newest of several reports suggesting a role for this adaptor in these innate effecter cells. The phosphorylation of LAT has been reported for engagement of NK cells with target cells and it has been suggested to be an important target of SHP-1 in KIR-mediated regulation of cytolytic activity.14,33 Our finding that LAT participates directly in Ly49/DAP12 and KIR/DAP12 signaling provides an explanation for the variation in LAT phosphorylation and variable effects of LAT overexpression in different NK clones observed by Jevremonvic et al.33 These authors used the target cell line CIR, which expresses HLA-Cw4. We found that HLA-Cw4 expressing target cells were more efficient in the induction of LAT phosphorylation, and we were unable to induce phosphorylation of LAT in fresh NK cells with MHC class I–deficient targets. Together, these data support the conclusion that some, if not all, target cell–induced LAT phosphorylation is mediated via KIR-associated ITAMs. Thus variation in the activating KIR haplotypes of NK clones results in different levels of dependency on LAT.
Our data are the first to demonstrate directly the use of LAB in NK-cell ITAM signaling. Expression of this adaptor has been demonstrated in human NK cells, but until now its subcellular localization and direct biochemical involvement were only implied by functional assays of murine NK cells.15 Our observation that the expression levels of some Ly49s are reduced in Lat−/− and/or Lab−/− NK cells is intriguing and may indicate a change in the Ly49 repertoire due to changes in NK-cell signaling. Interestingly, few signaling mutations have been reported that affect the development of the Ly49 repertoire. Most dramatic among these are the mutations of PLCγ2 or the Axl/Tyro/Mer receptors that result in profound reductions in Ly49 expression due to an apparent arrest in NK-cell development.34,35 As both LAT and LAB have been implicated in the regulation of PLCγ it is tempting to speculate that the changes in the Ly49 repertoire of LAT, LAB, and double-null mice are due to changes in their ability to regulate the activation of this key enzyme. It is also interesting to note that in resting NK cells, LAB mutation represents a “gain of function” mutation (see below), suggesting that increasing the signaling of NK receptors may influence the development of the Ly49 repertoire differently than loss of function mutations exemplified by Lat−/− mice.
Evidence for dual signaling cassettes in NK cells
In addition to defining a role for LAB in the regulation of NK-cell function, our findings suggest the existence of 2 parallel signaling pathways. The first, comprising ZAP70 and LAT, is clearly dominant in T cells. The strict dependency of T cells on this cassette is apparent due to the dramatic phenotype of Lat−/− and Zap70−/− mice and the relatively poor reconstitution of calcium mobilization of LAT− Jurkat lines by LAB.12,36–38 Although we have not directly demonstrated ZAP70 phosphorylation in response to ITAM signaling in Syk−/− NK cells, based on our findings here, we propose that the reason LAB only partially compensates for the lack of LAT in T cells might be due to a relative inability of ZAP70-mediated signals to crosstalk to LAB. Indeed, this hypothesis is consistent with the largely overlapping expression patterns of the members of the second cassette, Syk and LAB. In contrast to the monogamous use of LAT by ZAP70, a Syk-mediated cassette may readily utilize either LAT or LAB. Therefore, Lat−/− mast cells (which express Syk) maintain reduced but significant responsiveness, our Syk reconstituted, LAT low RNKDLS cells regain function, and resting Lat−/− NK cells still signal via ITAMs. Additional support for this model comes from reports demonstrating that some Syk-mediated signaling pathways are not effectively reconstituted by ZAP70.39,40
Interplay between LAT and LAB in NK cells
NK cells are not the only population to utilize both LAT and LAB. Mast cells also express both adaptors where they are involved in FcϵRI signaling.25,29,41 Based on our findings in resting NK cells it appears that some of the reported interplay between LAT and LAB in mast cells is recapitulated in the NK-cell compartment. Lat−/− mast cells show a significant reduction in FcϵRI responses, Lab−/− mast cells are hyperresponsive and double-null cells are largely nonresponsive.41 These data have been interpreted as defining a competitive interaction between LAT and LAB where the latter exerts a negative regulatory role, perhaps by limiting the amount of LAT that gains access to membrane Raft/glycolipid enriched membrane fractions. We were unable to detect a shift in the subcellular localization of LAT or LAB when one or the other was deleted (G.W. and D.W.M., unpublished results, June 2005). In contrast to mast cells, double-null NK cells had a profound defect in NK1.1-induced IFNγ but notably the response was not zero suggesting another, as yet undefined pathway for ITAM-mediated signaling.
Demonstration of reduced NK1.1 signaling in LAT−/− ALAK may appear at odds with the intact signaling we see in fresh NK cells. Here again, the requirement for efficient Syk signaling in a LAB-dominated cassette provides a compelling explanation. The rarely studied, cytokine-induced, adaptor mast cell immunoreceptor signal transducer (MIST)/cytokine dependent hemopoietic cell linker (CLNK) has recently been shown to provide positive signals in CD4 + NK/T cells but to exert inhibitory pressure on NK1.1 signaling in NK cells.42 These authors propose a mechanism whereby the Src-family kinase Fgr directly binds and down regulates Syk. In our cytokine propagated Lat−/− ALAK cells, MIST would be ideally positioned to suppress Syk. Indeed the MIST/CLNK-induced reduction in Syk activity of Lat−/− ALAK cells reveals greater dependency on LAT as predicted by our experiments in RNKDLS. Importantly, freshly isolated NK cells do not express significant MIST/CLNK and, therefore, we propose that their competent Syk signaling cassette permits efficient signaling via either LAT or LAB.43,44
Although use of both LAT and LAB has been suggested by functional analysis of NK cells, we are the first to provide direct biochemical evidence for the dual use of these proteins. In defining the interplay between these adaptors we find that contrary to previous reports, resting NK cells do recapitulate the apparent negative regulatory role of LAB described in mast cells.15 Furthermore, we provide mechanistic evidence of a Syk-LAB/LAT signaling cassette that circumvents the strict requirement for LAT in the ZAP70 cassette used in T cells. This complex and regulated adaptor protein configuration explains NK cells unique resilience to loss of 1 of the 4 key components and provides the plasticity to evolve functional novel receptor systems. Perhaps this is a reflection of the importance of dependable, rapid innate NK-cell activity during viral infection.
Supplementary Material
Acknowledgments
The 721.221 were from R. DeMars and the 721.221-Cw4 were from J. Gumperz and P. Parham. HP-3E4 was from M. Lopez-Botet. LAT−/− mice were kindly provided by Dr C. Summers and Dr L. Samelson.
This project has been funded in whole or in part with federal funds from the National Cancer Institute Intramural Research Program (Bethesda, MD).
The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.C.W. performed research, collected, analyzed, and interpreted data, and drafted the manuscript; D.N.B. designed research, collected, analyzed, and interpreted data, and drafted the manuscript; S.J.O. performed research and collected, analyzed, and interpreted data; L.Q. performed research and collected data; D.L.H. performed research and collected, analyzed, and interpreted data; V.P. collected, analyzed, and interpreted data; W.Z. contributed vital reagents; and D.W.M. designed and performed research, collected, analyzed, and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel W. McVicar, PhD, NCI-Frederick, Building 560, Room 31-46, Frederick, MD 21702; e-mail: mcvicard@mail.nih.gov.
References
- 1.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–259. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 4.McVicar DW, Taylor LS, Gosselin P, et al. DAP12 mediated signal transduction in NK cells: a dominant role for the Syk protein tyrosine kinase. J Biol Chem. 1998;273:32934–32942. doi: 10.1074/jbc.273.49.32934. [DOI] [PubMed] [Google Scholar]
- 5.Olcese L, Cambiaggi A, Semenzato G, et al. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J Immunol. 1997;158:5083–5086. [PubMed] [Google Scholar]
- 6.Brdicka T, Imrich M, Angelisova P, et al. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J Exp Med. 2002;196:1617–1626. doi: 10.1084/jem.20021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Irvin BJ, Trible RP, Abraham RT, Samelson LE. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 9.Janssen E, Zhu M, Zhang W, Koonpaew S, Zhang W. LAB: a new membrane-associated adaptor molecule in B cell activation. Nat Immunol. 2003;4:117–123. doi: 10.1038/ni882. [DOI] [PubMed] [Google Scholar]
- 10.Saitoh S, Arudchandran R, Manetz TS, et al. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- 11.Pasquet JM, Gross B, Quek L, et al. LAT is required for tyrosine phosphorylation of phospholipase cgamma2 and platelet activation by the collagen receptor GPVI. Mol Cell Biol. 1999;19:8326–8334. doi: 10.1128/mcb.19.12.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Sommers CL, Burshtyn DN, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 13.Galandrini R, Palmieri G, Piccoli M, Frati L, Santoni A. CD16-mediated p21ras activation is associated with Shc and p36 tyrosine phosphorylation and their binding with Grb2 in human natural killer cells. J Exp Med. 1996;183:179–186. doi: 10.1084/jem.183.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valiante NM, Phillips JH, Lanier LL, Parham P. Killer cell inhibitory receptor recognition of human leukocyte antigen (HLA) class I blocks formation of a pp36/PLC-gamma signaling complex in human natural killer (NK) cells. J Exp Med. 1996;184:2243–2250. doi: 10.1084/jem.184.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiesa S, Mingueneau M, Fuseri N, et al. Multiplicity and plasticity of natural killer cell signaling pathways. Blood. 2006;107:2364–2372. doi: 10.1182/blood-2005-08-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litwin V, Gumperz J, Parham P, Phillips JH, Lanier LL. Specificity of HLA class I antigen recognition by human NK clones: evidence for clonal heterogeneity, protection by self and non-self alleles, and influence of the target cell type. J Exp Med. 1993;178:1321–1336. doi: 10.1084/jem.178.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu Y, DeMars R. Production of human cells expressing individual transferred HLA-A,-B,-C genes using an HLA-A,-B,-C null human cell line. J. Immunol. 1989;142:3320–3328. [PubMed] [Google Scholar]
- 18.Malnati MS, Lusso P, Ciccone E, et al. Recognition of virus-infected cells by natural killer cell clones is controlled by polymorphic target cell elements. J Exp Med. 1993;178:961–969. doi: 10.1084/jem.178.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason LH, Willette-Brown J, Taylor LS, McVicar DW. Regulation of Ly49D/DAP12 signal transduction by Src-family kinases and CD45. J Immunol. 2006 doi: 10.4049/jimmunol.176.11.6615. In Press. [DOI] [PubMed] [Google Scholar]
- 20.Ortaldo JR, Mason AT, Winkler-Pickett R, et al. Ly-49 receptor expression and functional analysis in multiple mouse strains. J Leukoc Biol. 1999;66:512–520. doi: 10.1002/jlb.66.3.512. [DOI] [PubMed] [Google Scholar]
- 21.McVicar DW, Winkler-Pickett R, Taylor LS, et al. Aberrant DAP12 signaling in the 129 strain of mice: implications for the analysis of gene-targeted mice. J Immunol. 2002;169:1721–1728. doi: 10.4049/jimmunol.169.4.1721. [DOI] [PubMed] [Google Scholar]
- 22.Peruzzi M, Wagtmann N, Long EO. A p70 killer cell inhibitory receptor specific for several HLA-B allotypes discriminates among peptides bound to HLA-B*2705. J Exp Med. 1996;184:1585–1590. doi: 10.1084/jem.184.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirwan S, Merriam D, Barsby N, McKinnon A, Burshtyn DN. Vaccinia virus modulation of natural killer cell function by direct infection. Virology. 2006;347:75–87. doi: 10.1016/j.virol.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 24.Mackett M, Smith GL, Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984;49:857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu M, Liu Y, Koonpaew S, Granillo O, Zhang W. Positive and negative regulation of FcϵRI-mediated signaling by the adaptor protein LAB/NTAL. J Exp Med. 2004;200:991–1000. doi: 10.1084/jem.20041223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institutes of Health. Guide for the Care and Use of LaboratoryAnimals. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health; 1985. [Google Scholar]
- 27.Hodge DL, Martinez A, Julias JG, Taylor LS, Young HA. Regulation of nuclear gamma interferon gene expression by interleukin 12 (IL-12) and IL-2 represents a novel form of posttranscriptional control. Mol Cell Biol. 2002;22:1742–1753. doi: 10.1128/MCB.22.6.1742-1753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winter CC, Gumperz JE, Parham P, Long EO, Wagtmann N. Direct binding and functional transfer of NK cell inhibitory receptors reveal novel patterns of HLA-C allotype recognition. J Immunol. 1998;161:571–577. [PubMed] [Google Scholar]
- 29.Tkaczyk C, Horejsi V, Iwaki S, et al. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and Fc epsilon RI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- 30.Arase N, Arase H, Park SY, et al. Association with FcRgamma is essential for activation signal through NKR-P1 (CD161) in natural killer (NK) cells and NK1.1+ T cells. J Exp Med. 1997;186:1957–1963. doi: 10.1084/jem.186.12.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell KS, Cella M, Carretero M, Lopez-Botet M, Colonna M. Signaling through human killer cell activating receptors triggers tyrosine phosphorylation of an associated protein complex. Eur J Immunol. 1997;28:599–609. doi: 10.1002/(SICI)1521-4141(199802)28:02<599::AID-IMMU599>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Swat W, Fujikawa K. The Vav family: at the crossroads of signaling pathways. Immunol Res. 2005;32:259–265. doi: 10.1385/IR:32:1-3:259. [DOI] [PubMed] [Google Scholar]
- 33.Jevremovic D, Billadeau DD, Schoon RA, et al. Cutting edge: a role for the adaptor protein LAT in human NK cell-mediated cytotoxicity. J Immunol. 1999;162:2453–2456. [PubMed] [Google Scholar]
- 34.Tassi I, Presti R, Kim S, et al. Phospholipase C-gamma 2 is a critical signaling mediator for murine NK cell activating receptors. J Immunol. 2005;175:749–754. doi: 10.4049/jimmunol.175.2.749. [DOI] [PubMed] [Google Scholar]
- 35.Caraux A, Lu Q, Fernandez N, et al. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat Immunol. 2006;7:747–754. doi: 10.1038/ni1353. [DOI] [PubMed] [Google Scholar]
- 36.Negishi I, Motoyama N, Nakayama K, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 37.Koonpaew S, Janssen E, Zhu M, Zhang W. The importance of three membrane-distal tyrosines in the adaptor protein NTAL/LAB. J Biol Chem. 2004;279:11229–11235. doi: 10.1074/jbc.M311394200. [DOI] [PubMed] [Google Scholar]
- 38.Janssen E, Zhu M, Craven B, Zhang W. Linker for activation of B cells: a functional equivalent of a mutant linker for activation of T cells deficient in phospholipase C-gamma1 binding. J Immunol. 2004;172:6810–6819. doi: 10.4049/jimmunol.172.11.6810. [DOI] [PubMed] [Google Scholar]
- 39.Zoller KE, MacNeil IA, Brugge JS. Protein tyrosine kinases Syk and ZAP-70 display distinct requirements for Src family kinases in immune response receptor signal transduction. J Immunol. 1997;158:1650–1659. [PubMed] [Google Scholar]
- 40.Latour S, Chow LL, Veillette A. Differential intrinsic enzymatic activity of Syk and Zap-70 protein-tyrosine kinases. J Biol Chem. 1996;271:22782–22790. doi: 10.1074/jbc.271.37.22782. [DOI] [PubMed] [Google Scholar]
- 41.Rivera J. NTAL/LAB and LAT: a balancing act in mast-cell activation and function. Trends Immunol. 2005;26:119–122. doi: 10.1016/j.it.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Sasanuma H, Tatsuno A, Hidano S, et al. Dual function for the adaptor MIST in IFN-γ production by NK and CD4+NKT cells regulated by the Src-kinase Fgr. Blood. 2006 doi: 10.1182/blood-2005-10-4102. [DOI] [PubMed] [Google Scholar]
- 43.Goitsuka R, Tatsuno A, Ishiai M, Kurosaki T, Kitamura D. MIST functions through distinct domains in immunoreceptor signaling in the presence and absence of LAT. J Biol Chem. 2001;276:36043–36050. doi: 10.1074/jbc.M106390200. [DOI] [PubMed] [Google Scholar]
- 44.Utting O, Sedgmen BJ, Watts TH, et al. Immune functions in mice lacking Clnk, an SLP-76-related adaptor expressed in a subset of immune cells. Mol Cell Biol. 2004;24:6067–6075. doi: 10.1128/MCB.24.13.6067-6075.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.