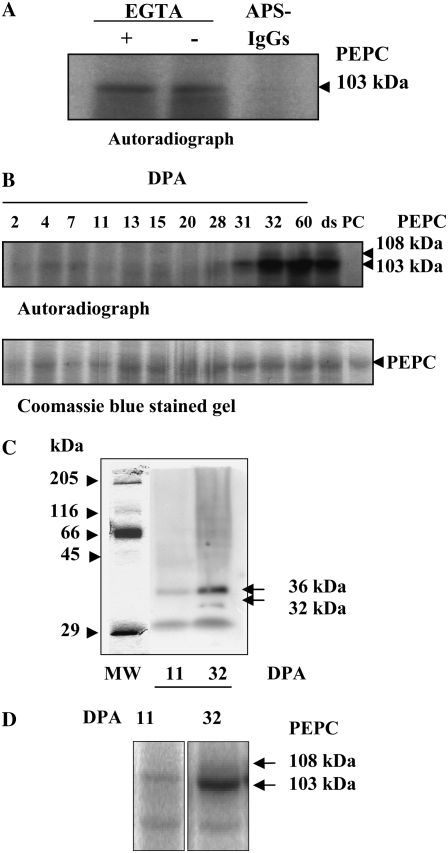
Figure 4.
Characterization of the PEPC kinase. A, Effects of EGTA and APS-IgGs on in vitro PEPC kinase activity. Aliquots of desalted extracts from whole dry seeds (150 μg of total protein, containing 0.02 units of endogenous PEPC) were incubated at 30°C for 1 h in the presence of components of the phosphorylation assay lacking sorghum PEPC and 1 mm EGTA or 40 μg of APS-IgGs (APS-IgGs). B, PEPC kinase activity profile during the development of barley seeds. Aliquots of desalted extracts from seeds harvested at different stages of development (0.02 units of endogenous PEPC) were incubated at 30°C for 1 h in the presence of 0.08 units of purified, nonphosphorylated sorghum leaf PEPC as an exogenous substrate and the components of the phosphorylation assay. The in vitro phosphorylation reaction was stopped by the addition of dissociation buffer, pH 6.8, and heat denaturation. Proteins were separated by SDS-PAGE (10% acrylamide) and analyzed by autoradiography. ds, Dry seed; PC, 0.08 units of purified, nonphosphorylated sorghum PEPC. C, In gel assay of PEPC kinase activity from crude extracts at two stages (11 and 32 DPA) of seed development. Soluble proteins from crude extracts (500 μg) were separated by SDS-PAGE (15% acrylamide). After elimination of SDS, proteins were renatured as described in “Materials and Methods,” then the gel was incubated in the presence of [γ-32P]ATP (50 μCi per 15 mL) and 10 mm MgCl2 and analyzed by autoradiography. D, The experiment was performed as described in B, in the absence of exogenous PEPC and using concentrated aliquots containing 250 μg of protein.